Astragalus, Astragaloside IV
#811
Posted 30 July 2010 - 10:07 PM
An answer during the Q&A reassured me somewhat about my reservations about telomerase activation. (They also clarified that TAT153 is "an orally available proprietary molecule". Recall that some versions of Anthony's Astral Fruit product contain a naturally derived molecule that Geron called TAT2. Both have shown promise in activating telomerase in human cells in vitro. TAT153 is the one that they're investing in for preclinical studies toward an IND candidate for human trials.) Geron's theory is the same thing Anthony has been saying and that speaker in the TA Sciences video stated: Geron believes that the mechanism of telomerase activation from TAT153 [and TAT2, I presume] is completely different from what happens with cancer. There's "zero evidence that chronic exposure" to the drug candidate causes cancer. The effect is reversible -- telomerase activity "goes back to zero within 48-72 hours of withdrawal" of the drug. It seems to act on already "telomerase competent" cells, basically the "resident adult stem cells". This is different from TA that occurs during late stages of epithelial cells turning into cancer cells. In cancer, changes in growth control occur first, and then telomerase is permanently turned on. However, it's a "very valid concern" and they plan to do more research, but so far there's no evidence so far that there's a danger in activating telomerase [to cure disease].
#812
Posted 31 July 2010 - 06:35 AM
Mark Monane – Needham & Company
That is helpful. And then allow me one more question on the TAT program. We live in a world that is full of [telomerase] inhibition and blockers, but here you are using an activator, and I guess the question for those cyclist enthusiasts out there, how would it be possible to have a break in this program, a break in the clinical trial and to potentially offset any unintended side effects using the activator program, which is truly much more ambitious than the inhibitor program?
Thomas Okarma
First, we have already done quite a bit of testing of this drug in tumor induction models, and there is absolutely zero evidence that chronic exposure of this agent in those tumor induction models has any impact on the frequency of tumor induction, and that is consistent with the mechanism. This is a reversible pharmacologic induction of Telomerase in so-called Telomerase-competent cells, which are more or less the so-called resident adult stem cells in organs that self-renew throughout life like skin, the lining of the gut, and bone marrow. And it is reversible. So, it goes back, the Telomerase activity goes back down to zero within 48 or 72 hours of withdrawal of the drug. That mechanism is quite different from the constitutive permanent upregulation of Telomerase that occurs late in the process of malignant transformation. So, as cells go from the normal epithelial category to a malignant line, there are multiple genetic abnormalities in growth control, which precede the constitutive permanent upregulation of Telomerase activity. So, the mechanisms are quite different and again we have not, although it’s a very valid concern, and we will do more of this kind of work, we see no evidence that TAT upregulation with 153 would have any impact on inducing malignancy. It’s a good question, Mark. Thank you.
So that seems to support Anthony's reassurances based on very early work published by Geron scientists: their latest research still shows that telomerase activation with one of these molecules won't turn on telomerase in a cell that is about to become malignant, and unlike with cancer, when you stop taking the pills, the cells that did turn it on soon turn it back off.
That should be reassuring to those of us optimistically taking natural supplements hoping for health benefits. For the FDA's standard of safety, I'm sure Geron will have to do a lot of preclinical research showing safety of TAT153 and that it will be one of the things they look at in the Phase I safety trial someday if they get that far.
#813
Posted 03 August 2010 - 06:03 AM
sponsored ad
#814
Posted 03 August 2010 - 04:28 PM
I read the whole thing. So does Cycloastragenol work or not?
The drugs by geron and natural chemicals in Cycloastragenol may very well activate telomerase as advertised but we do not know if this is maintaining telomere length with out tools to track individuals cells through out their lifetime in the human body. They can only make very poor guestimates based on fish flow analysis over 10 year periods.
There are other factors to telomere maintenance besides telomerase activation. According to a study I read recently, telomeres are not always in a state to accept telomerase. Simply having more telomerase does not increase the chance telomeres will accept the enzyme. Along those same lines, anyone know how telomerase is actually delivered to cells from the stomach?
It is believed that telomeres switch between two states: capped and uncapped. The telomere state determines its accessibility to telomerase and also the onset of senescence.
http://www.pnas.org/...387.short?rss=1
Edited by bsm, 03 August 2010 - 05:03 PM.
#815
Posted 07 August 2010 - 09:25 AM
Then I'll wait spending $$$ till a positive result is assured.
#816
Posted 07 August 2010 - 02:17 PM
sounds like a reasonable decision. Just like I mentioned in an old post of mine here, I would ask most folks that are on the fence, to simply wait for more studies. Telomeres shrink slowly, and unless you are of an advanced age or are an early adopter who has considerable knowledge to make a solid educated decision, then I would ask most to take their time to consider such a supplement.
This thread, (as long as it is) is the most informative I have been involved with anywhere on the internet. (Well maybe except for a couple of Dr V's tidbits in our newsletter).
But I believe that most relevant information will be provided here, regarding the latest findings, products and studies. Folks that are not on the fence and taking some sort of telomerase activator, can also keep watching this topic by clicking the link below, I think it is a nifty function that this forum provides.
I invite you to keep up to date by clicking this link to be notified when new posts are available:
http://www.imminst.o...=list&tid=19921
Cheers
A
Edited by Anthony_Loera, 07 August 2010 - 02:26 PM.
#817
Posted 11 August 2010 - 06:34 PM
Had he mentioned and talked?:
http://www.imminst.o...ost&f=6&t=19921
http://www.sciencedi...504075a74fd08d2
http://www.ncbi.nlm....pubmed/12586694
Does anyone know if the tea Roiboos SOD does something or destroyed in the stomach?
Have you noticed in the power of chitosan to cancel peroxidation superoxide and also of its power to repair cells in that article?
"Melatonin should be taken separately from other activators of telomerase to be a compound related to the dream?
How telomerase activity should include activators of p53 or interacting with him?
Is there enough to earn a few units of telomeres at each outlet not exceeding the number that are lost by replication of suicide for telomerase activity safe?
Is there any measurement of telomerase activity in derivatives of portulaca oleacea compared to others?
Is the there with tryptophan (or portulaca oleacea -verdolaga-), rosavin (root of rhodiola rosea), and melatonin (So, possibly ways to get segregate ephitelion -Are there more candidates?-) compared with cycloastragenol?
Is there something about interactions between all?
I think there's a 2007 patent on the formula at least one type telomerase activator cycloastragenol. But can not find it now. Does anyone know it? Can you explain the new compounds of GEROM?
Does the repair capacity of chitosan could lower the activity of telomerase in cell repair?
If so could be good for those cells, neutral for other cells, and very good and suitable for nerve cells?
Shilima khemen
#819
Posted 11 August 2010 - 06:51 PM
*****
resveratrol helped counteract changes in SIRT2 expression induced by a high-calorie diet
******
?¿
http://www.htrf.com/...CFdeX2AodP3Xd5Q
Shilima khemen
#820
Posted 15 August 2010 - 01:15 PM
As I said in another time in this blog, the telomeres are transcribed into RNA, this RNA is called telRNA or TERRA.
The telARN indicate the actual length of telomeres
When telomerase is activated only gain a number of telomere units according to telARN
And one who is in control milARN290
Therefore, in addition to telomerase activity would require a regulatory substance to control the number of units generated instead of taking more of a substance or more times and the difficulties that have been in this thread on the low activity telomerase activators without cancer risk
Well. A new article in Nature of Maria Blasco and others (open access to the full article):
http://www.nature.co...ncomms1032.html
It shows how certain proteins control a number of units won (hnRNP A1, hnRNP A2B1, hnRNP F and hnRNP M) and how to adjust your control over telomere length gained
I think is quite relevant and very important in this.
This means that along with the activator (or activators of telomerase) should add a control substance in length and may vary this as they move takes
GEROM know this? Explain changes in amounts of the TA64? Does the TA64 would be a union between activator and regulator of telomere length? Anything else? None?
And, in "Nature Cell Biology" another study is published shelterines proteins (TRF1, TRF2, POT1, RAP1, TIN2, and TPP1)
Proteins that form a protective shield at the end of the telomere (not essential, but with relevance) that are encoded elsewhere in the chromosome with functions related to carcinogenic processes
Shilima khemen
#821
Posted 15 August 2010 - 06:57 PM
Maria Blasco's work in the past has been pretty cutting edge, and solid.
She is personally one of my top 10 scientist all-stars regarding telomere work.
There are a lot of things in her findings that are interesting, however downregulation of hnRNP M and D seem to be most interesting as she states that it "strongly increased the mobilization of TERRA to telomeres".
In my opinion, I don't think 'Gerom' is at this level of investigation, however her paper may produce new avenues of investigation for us and others.
A
#822
Posted 16 August 2010 - 07:07 PM
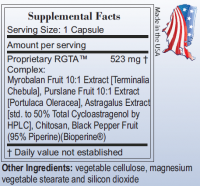
I'm excited about your new product, but what is exactly in Astral Fruit-NF RGTA™ Complex other than 5mg cycloastragenol?
#823
Posted 16 August 2010 - 07:43 PM
Terminalia Chebula
Portulaca Oleracea
Astragalus Extract With Cycloastragenol
we have also added chitosan, bioperine.
This is our proprietary complex in Astral Fruit-NF for telomerase support.
60 Capsules, each capsule runs about 500mg.
Please review the following if you are not familiar with some of these:
PubMed ID: 15478203
PubMed ID: 17764668
PubMed ID: 18981163
We expect this product to perform very well compared to others telomerase activators.
Edited by Anthony_Loera, 16 August 2010 - 07:44 PM.
#824
Posted 16 August 2010 - 07:55 PM
How much Cycloastragenol is in this formulation?
With a "proprietary formulation" it could be 0.0001mg and you would not be breaking the law. Please specify at least how much cycloastragenol you have, even if for some reason you wish to keep the proportions of the other ingredients secret.
If you don't reveal the quantity of cycloastragenol, skeptics will probably say that the reason you aren't revealing it is because you have such a minimal quantity that people wouldn't buy it if they knew the amount.
Fess up.
#825
Posted 16 August 2010 - 08:20 PM
#826
Posted 16 August 2010 - 08:37 PM
This is growing in my windowbox.Portulaca Oleracea
#827
Posted 17 August 2010 - 12:26 AM
I will confirm that your daily intake will contain 5mg or more of cycloastragenol with the new product.
Cheers
A
Edited by Anthony_Loera, 17 August 2010 - 03:22 AM.
#828
Posted 17 August 2010 - 03:22 AM
Yes I will confirm that your daily intake will contain 5mg or more of cycloastragenol with the new product.
Before you edited your 1st response, it said that there was 5mg if you took 3 capsules per day. Not sure why you deleted that info.
So what you're saying is that you took a product which was 30 capsules with 5mg each of cycloastragenol, and now you're selling a different product which is 60 capsules but you have to take 3 to get 5mg of cycloastragenol?
It sounds like you've just bumped up the price by 50%.
Edited by smithx, 17 August 2010 - 03:23 AM.
#829
Posted 17 August 2010 - 03:30 AM
Nope, I can happily state that you are getting the exact amount of cycloastragenol or more than our last formulation.
Which means that you are getting the other two telomerase support ingredients for practically... free?
Wasn't there the word more in my first response? The reason it was edited was because I wrote it from my phone, and made a mistake. Since I could not edit it, I wrote another post from my phone. And... What does that email say?
Don't you get email notifications with the original posts?
If you do, you will see my initial post error, and my original second post stating that it was not possible to edit a post on my phone.
Cheers
A
(I must have edited this particular post over 7-8 times.. oof!)
Edited by Anthony_Loera, 17 August 2010 - 03:52 AM.
#830
Posted 17 August 2010 - 08:04 AM
Nope, I can happily state that you are getting the exact amount of cycloastragenol or more than our last formulation.
OK, so am I (and others) misunderstanding that there was 5mg of cycloastragenol in each capsule of your previous formula? If so, based on your most recent statement, there is at least 5mg of cycloastragenol in each capsule of your current formula, correct?
None of this dancing around would be needed if you would just state the quantities.
#831
Posted 17 August 2010 - 01:24 PM
it is more complicated than that, as we now have 60 capsules and a recommendation to take 1 capsule with each meal for when you are cycling through this product. This is not a two month supply with 60 capsules, this is a one month supply with 60 capsules. In our last formulation with 30 capsules, you where getting 150mg for the whole month. With this formulation you are getting the same or more than that.
Smithx, I know you want our exact formulation, but because it is a trade secret it's simply not going to happen. You are simply trying to take advantage of my good nature here, because I actually respond to questions... while other companies never do.
Tell you what, if you can get the ta sciences folks to tell you why they now only use astragalus root extract (then calling it ta-65* per their new label) which we know can be found anywhere, that would be great. Remember according to FDA rules (that are used to prevent adulteration of supplements) we can see that from their new label alone, that they are not using cycloatragenol, astragaloside IV, or other pure telomerase activators in their product. In fact according to the FDA, if they did use these in the product they would have to be clearly labeled in the supplement section, as we had to do in our own label. Of course we don't see any of this in the attached label of theirs. Since we assume that these folks are following FDA rules, then they are clearly only selling astragalus root extract to folks, and not anything out of the ordinary that cannot be found in your local supplement shop.
So smithx, if you can get some good answers from ta sciences... Then maybe, just maybe, I may consider providing you my numbers for cycloastragenol in our current product. As far as I know, we are the only supplement manufacturer that currently offers supplements that have Astragalus with a verified amount of Cycloastragenol.
Cheers
A
Competitor comparisons are welcomed:
Compare both labels below, the first one is from ta sciences inc, and the other one is ours. According to FDA label regulations, which one actually provides cycloastragenol?
You can compare the labels for themselves. This is simply considered a truthful and verifiable comparison of two competitor labels and is allowed by law, and encouraged.
The first label here shows that TA-65* is the proprietary name for "Astragalus Root Extract" in our competitor's label, while RGTA is our proprietary name for Terminalia Chebula, Portulaca Oleracea, Astragalus Extract With Cycloastragenol, chitosan, and Bioperine complex.
*TA-65 is a trademark registered to Telomerase Activation Sciences, Inc.
Myself, RGTA and my company RevGenetics are in no way associated with Telomerase Activation Sciences, Inc.
These statements in this post have not been evaluated by the Food and Drug Administration. These products are not intended to diagnose, treat, cure, or prevent any disease.
Edited by Anthony_Loera, 17 August 2010 - 02:32 PM.
#832
Posted 17 August 2010 - 08:48 PM
Edited by mrak1979, 17 August 2010 - 08:49 PM.
#833
Posted 17 August 2010 - 11:33 PM
Tell you what, if you can get the ta sciences folks to tell you why they now only use astragalus root extract (then calling it ta-65* per their new label) which we know can be found anywhere, that would be great. Remember according to FDA rules (that are used to prevent adulteration of supplements) we can see that from their new label alone, that they are not using cycloatragenol, astragaloside IV, or other pure telomerase activators in their product. In fact according to the FDA, if they did use these in the product they would have to be clearly labeled in the supplement section, as we had to do in our own label.
I'm not in any way anti-you or anti-revgenetics, but I do prefer companies I deal with to be open and honest.
With regard to ta sciences - they are using ta-65 which has been researched and found to actually work at least to some extent. Your product doesn't have the same research, so buying a black box isn't the same proposition. And I don't buy anything from ta sciences anyway.
Further, as I understand it, if you use an extract of some herb you are under no obligation to disclose that it is standardized to some quantity of whatever or that it was extracted using whatever method. Those are optional. All that you have to disclose is that you're using an extract of whatever GRAS herb you're using. If you know the rules to state otherwise, it would be instructive to be linked to the FDA regulations which specify this.
So to recap, your previous formulation had 5mg of cycloastrogenol per capsule but your current formulation has about 2.5mg per capsule or perhaps very slightly more? Is that or is that not a fair statement.
#834
Posted 18 August 2010 - 01:17 AM
Further, as I understand it, if you use an extract of some herb you are under no obligation to disclose that it is standardized to some quantity of whatever or that it was extracted using whatever method. Those are optional. All that you have to disclose is that you're using an extract of whatever GRAS herb you're using. If you know the rules to state otherwise, it would be instructive to be linked to the FDA regulations which specify this.
They are not doing anything illegal if all they are doing is claiming their product has nothing more than astragalus root extract, and calling it by another name. It however it leaves many issues open for consumers to consider:
The facts remain:
1- I know what cycloastragenol did in Geron's UCLA study, and again when our own material was tested.
2- We require standardized astragalus with measurable amount of cycloastragenol, other companies do not.
3- You don't know what material was tested as ta-65 (And, why change the formulation if the 'studies' where successful?)
4- According to the label they adhere to no standardized astragalus root extract, this simply means that they can provide the best or the worst quality without notifying you of any change in quality. I believe folks want purity and consistency they can count on, and not rely on a manufacturer that can change it on a whim.
5- Without standards, or proven purity levels, you cannot be assured that one batch is different than another in manufacturing. Again, is this what you want smithx or do you want to be assured of some sort of standardization?
The point of labeling a special active:
By placing the active ingredient on the label, the company assures the active ingredient is in the product. It serves as a measure of trust for the customer, and allows the customer to have recourse with the FDA if he believes the ingredient is not found in the product, and is adulterated or mislabeled. Since we place the active ingredients on the label, we are not only assuring our customers that we use them in the product, but that the FDA can hang us if they are not found in the product.
This sounds familiar:
This again reminds me a lot of the resveratrol craze, where people stated that a 'grape extract' supplement had resveratrol in it, but none was found in the label. People assumed it could have some resveratrol and bought it, but never knew how much. After it came out that grape extract had little to no resveratrol was not standerdized, etc... we started looking at folks who started to demand actual resveratrol to be placed on the labels.. then when cis and trans form of resveratrol was understood, we found folks wanting to make sure that their product had "Trans-resveratrol" on the label. Then the requests for high purity started coming in, and we produced 98% and 99% pure resveratrol and micronized res for the more demanding customers.
Today, I still walk in to Wallgreens and walmart and see "Grape Extract" pills on sale for the same price we sell 50% and 98% pure resveratrol. and think "Wow, people still are throwing their money away?" But hey, it's an educational process, I realize that... so I hope this helps you smithx.
As Ronald Reagan used to say:
Trust, but verify... ok?
Cheers
A
Edited by Anthony_Loera, 18 August 2010 - 01:20 AM.
#835
Posted 18 August 2010 - 06:30 AM
For example, Abkit Kira St. John's wort extract (http://www.iherb.com...blets/3410?at=0) makes the following claims and does not state what it was standardized for:
* The Most Clinically Tested Formula In the World
* Exclusive LI160 Clinically Proven Formula
* From the Makers of Kwai Garlic
* Maintain A Healthy Emotional Balance With The World's Most Proven Formula
* Dietary Supplement
* Standardized and Tested
Kira Promotes A Healthy Emotional Balance & Well Being.
* Kira's advanced LI 160 Formula contains all of the essential constituents in this proven combination to unlock the benefits of St. John's Wort. Every Kira tablet is formulated to deliver the right dose that gets results, with the proven amounts of hyperforin, hypericin and flavonoids. Kira's ingredients work together to support a healthy balance among the brain's chemical messengers to promote a feeling of well-being. Other brands may try to make generic St. John's Wort claims based on Kira's extensive scientific research, but only Kira's LI 160 Formula has been proven in over 23 clinical studies.
The label only states:
St. John's Wort Extract (flower & leaves) (Hypericum perforatum L.)
So again, I think it is perfectly legal to make claims and have a proprietary extract, without stating exactly how you extract it or what you are standardizing for. Again, if you know otherwise, a link to the regulations would be useful.
This is all besides the point, however, which was that I'm still hoping for an answer to my previous question:
So to recap, your previous formulation had 5mg of cycloastrogenol per capsule but your current formulation has about 2.5mg per capsule or perhaps very slightly more? Is that or is that not a fair statement.
So is it?
Edited by smithx, 18 August 2010 - 06:39 AM.
#836
Posted 18 August 2010 - 01:40 PM
So I will hold your hand this time,
and sit you down... and explain it one last time:
Now concentrate please...
no, no, no... don't look over there, please focus and try to concentrate.
Ready?
Now look at the following sentence and then close your eyes, and let the words linger...
By providing generalized information in the supplement facts section, Abkit ALSO has the option of providing the best or the worst material in the world without notifying you of any change in quality.
Now... let that sink in.
Wait a moment, repeat it again if it doesn't make sense...
let it sink in.
Breath in
Breath out Danielsan...
wait, wait, no questions.. and put down your hand.
We are not done yet.
Ok... ready for another one?
I know, i know... it's boring stuff...
but try to concentrate please, as it may help you read labels better in the future.
All I am doing is trying to help regardless of the product you are buying...
No fidgeting,
no...don't look down at your shoes, we have another sentence to read.
Ok, ready?
Read the following sentence aloud please:
Folks that state actives on the label, do not have the option of shortchanging you on a whim. If it's on the label, it needs to be in the product.
Let that sink in please...
If you don't understand it, please repeat the sentences above. I know, it sometimes takes time. It is possible may still not understand, but don't worry... when the student is ready, the teacher will appear. So it is certainly possible that I am just a very lousy teacher, and you simply need someone closer to you to explain it and argue it out with.
Is the message getting across now?
I hope so... because I am pretty much done trying to explain this to you.
Lastly, in regards to the formulation, you can make any inference you like from my previous posts.
Cheers!
A
Edited by Anthony_Loera, 18 August 2010 - 03:06 PM.
#837
Posted 18 August 2010 - 05:19 PM
Smithx, it appears that you still don't understand even though you appear to be a smart person,
So I will hold your hand this time,
and sit you down... and explain it one last time:
Now concentrate please...
no, no, no... don't look over there, please focus and try to concentrate.
Ready?
Now look at the following sentence and then close your eyes, and let the words linger...By providing generalized information in the supplement facts section, Abkit ALSO has the option of providing the best or the worst material in the world without notifying you of any change in quality.
Now... let that sink in.
Wait a moment, repeat it again if it doesn't make sense...
let it sink in.
Breath in
Breath out Danielsan...
wait, wait, no questions.. and put down your hand.
We are not done yet.
Ok... ready for another one?
I know, i know... it's boring stuff...
but try to concentrate please, as it may help you read labels better in the future.
All I am doing is trying to help regardless of the product you are buying...
No fidgeting,
no...don't look down at your shoes, we have another sentence to read.
Ok, ready?
Read the following sentence aloud please:Folks that state actives on the label, do not have the option of shortchanging you on a whim. If it's on the label, it needs to be in the product.
Let that sink in please...
If you don't understand it, please repeat the sentences above. I know, it sometimes takes time. It is possible may still not understand, but don't worry... when the student is ready, the teacher will appear. So it is certainly possible that I am just a very lousy teacher, and you simply need someone closer to you to explain it and argue it out with.
Is the message getting across now?
I hope so... because I am pretty much done trying to explain this to you.
Lastly, in regards to the formulation, you can make any inference you like from my previous posts.
Cheers!
A
I don't think SmithX warranted being treated like an idiot. Why so absuive to people lately?
Edited by mikeinnaples, 18 August 2010 - 05:22 PM.
#838
Posted 18 August 2010 - 05:27 PM
So I will hold your hand this time,
You're getting really offensive. Why is that? Do you feel threatened?
Abkit in their marketing claims is stating that they are providing the customer with a particular formula which has been tested in 23 clinical studies. They do NOT have the option of changing the composition of their product unless they also stop saying that they are offering this specific formula.
You, on the other hand, can change whatever you want because you are not specifying any particular formula nor are you specifying any quantities. So it's your product which is suspect by your own reasoning.
It seems that you've gone from offering a product with a specified quantity of a specified compound, to offering a much more expensive product with no quantity specifications of any kind. I don't see that as an improvement.
I was looking forward to purchasing this product. All I wanted to know was the quantity of cycloastrogenol. Instead I receive evasive and then abusive responses. This does not inspire confidence nor does it make me want to order your products. There also appears to be no logical reason for you not to disclose the amount of cycloastrogenol except if you are trying to hide the fact that there isn't much in there.
Once again, my question is: what is the minimum quantity of cycloastrogenol in your product? You were alluding to it being at least 2.5mg per capsule. Do you guarantee it is at least that quantity, or are you not even willing to go on record stating that your product contains that minimal amount?
Edited by smithx, 18 August 2010 - 05:38 PM.
#839
Posted 18 August 2010 - 05:48 PM
I don't think SmithX warranted being treated like an idiot. Why so absuive to people lately?
Mikeinnaples,
The concept was being explained multiple times. Don't take my comment to smithx personally. But to clear things up on this thread, Mike do you agree with smithx's argument stating that supplement standards and actives are not important in labels to maintain consistent quality standards?
Smithx,
marketing claims, and fda label claims are two different things. Label claims must be adhered to or you get hanged by the FDA, while marketing claims are much more lenient as we have seen.
Basically, who cares about the marketing claims for a particular batch, if they can change their formulation tomorrow and never tell you. They could do this legally, without notice as long as claims are not placed directly on the label. That is the point of my argument.
I already stated the minimums that our bottled product carries here. There is no reason why you should keep asking for more information about a trade secret formulation. If you want to act like a troll on this thread, that is your prerogative. Don't expect me to be nice while you are trolling around.
A
I just read your deleted comment Mike:
Simply telling him that the formulation is proprietary and you do not which to divulge that information would suffice sans the abuse.
As you know, I did say that early on, but he still asks... and is trying to make a case that standards and purity do not matter. He is obviously no FDA lawyer, and certainly is not in the business himself.
Edited by Anthony_Loera, 18 August 2010 - 08:50 PM.
#840
Posted 18 August 2010 - 07:12 PM
I just read your deleted comment Mike:
Simply telling him that the formulation is proprietary and you do not which to divulge that information would suffice sans the abuse.
As you know, I did say that early on, but he still asks... and is trying to make a case that standards and purity do not matter. He is obviously no FDA lawyer, and certainly is not in the business himself.
Aside from my inability to spell 'wish' ...I deleted that post for a reason. Regardless, I can understand how someone would question the label because there is no real way of knowing how much of a certain product is in it. I can also understand his concern about buying something for cycloastrogenol content, when the content itself is not mentioned in any specific quantitiy. Your product is standardized to 50% cycloastrogenol content in the Astralagus extract, however it does not state the amount of the extract specficially. Since the formula is proprietary, you also have to option to change that formula. So the first time he buys the product, it may have 10mg of the Astralagus Extract ...who's to say, from a consumer perspective, that the formula won't change and instead have, lets say, 1mg later? He would be getting 1/5th the cycloastrogenol content that he wants without the label on the product ever having to change and without being in violation of any rules because it will still contain what it claims, just at different levels.
So yeah Anthony, I can understand where he is coming from ....and no Anthony, I don't think he warranted the comments and treatment from you. Even if he was completely off base and just trying to get a rise out of you, this is a public forum full of your customers and potential customers and it is never wise to exhibit anything but strictly professional behavior. I shouldn't have to tell you that.
Edit:
The point of labeling a special active:
By placing the active ingredient on the label, the company assures the active ingredient is in the product. It serves as a measure of trust for the customer, and allows the customer to have recourse with the FDA if he believes the ingredient is not found in the product, and is adulterated or mislabeled. Since we place the active ingredients on the label, we are not only assuring our customers that we use them in the product, but that the FDA can hang us if they are not found in the product.
Where is the quantity included in this? That, I believe, is the concern and the 'point'.
Edited by mikeinnaples, 18 August 2010 - 07:17 PM.
0 user(s) are reading this topic
0 members, 0 guests, 0 anonymous users































 This topic is locked
This topic is locked