The CRISPR equivalent of tool-and-die specialists are taking gene editing to the manufacturing scale.

Inscripta is a gene editing technology company that develops cell engineering tools. For example, the company provides MADzymes, which are proprietary versions of CRISPR enzymes that have special features such as different protospacer adjacent motif (PAM) recognition sequences, different cut efficiencies, reduced sizes, and different enzyme kinetics.
Research is being revolutionized by CRISPR; industry, not so much. As far as industry is concerned, the CRISPR approach to gene editing is still too rough and ready, suitable for tinkerers, perhaps, but lacking the sophistication needed in commercial applications, especially those meant for the clinic. For example, CRISPR is still hampered by licensing uncertainties and technological shortcomings, including delivery constraints, off-target activities, and poor scalability.
To help CRISPR realize its commercial potential, companies around the world are busily adding tools to the CRISPR toolbox, tools that offer ease of use, power, and sophistication—as well as economy. Many of these companies are newly founded startups. What they lack in longevity, they make up for with innovation. To catch up with what’s new and novel, GEN spoke with several CEOs of CRISPR-upgrading startups. Here’s what they told us.
High-throughput genome engineering
Unless better CRISPR tools are developed, CRISPR gene editing will have difficulty establishing commercial applications, suggests Kevin Ness, PhD, CEO, Inscripta. The company is developing a high-throughput, multiplexed genome editing platform that will, Ness asserts, allow scientists to perform tens of thousands of insertions, deletions, and swaps in one experiment.
“The traditional CRISPR/Cas9 approach performs one edit, querying one area of the genome, one edit at a time,” Ness notes by way of contrast. “That’s a very limited, laborious way to interrogate a genome or to create a specific cell.”
Inscripta’s genome engineering technology is meant to give users a fully automated benchtop system. It will be introduced later this year.
“The platform is based on a new chemistry,” Ness details. “The guides to cutting the genome and the homology arms that repair the cut are covalently attached to each other to create a large, precise cell library for genome engineering. This approach also barcodes the changes in each cell. Because the guide and homology arm are delivered simultaneously, scientists can precisely target many locations in a very scalable way.”
The platform combines Inscripta’s patented enzyme systems (MADzymes), proprietary chemistry, microfluidic-based instrumentation, and novel software and algorithms.
Historically, CRISPR access has been restricted by intellectual property rights and limitations related to efficiency, scalability, and costs. To help break the access barrier, Inscripta began providing the MAD7 enzyme for free to users in academia and commercial R&D.
“More than 1000 people downloaded the sequence in its first year,” Ness points out, adding that researchers have told him that single experiments with MAD7 have yielded results comparable to those obtained with Cpf1 (an alternative to Cas9). Additional MADzymes are planned for commercialization in the latter half of 2019.
“They can be used interchangeably for cutting and repairing the genome,” Ness asserts. MADzymes, coupled with Inscripta’s forthcoming high-throughput genome engineering technology, “will allow researchers to develop the most diverse and precise libraries possible, using iterative methods to engineer specific cell phonotypes.”
Targeted gene delivery
“CRISPR and gene editing tools are very powerful,” says Andre Watson, founder, chairman, and CEO of Ligandal. “But you still have to get the edits into the right cells.” Otherwise, gene editing won’t make the transition to gene therapy.
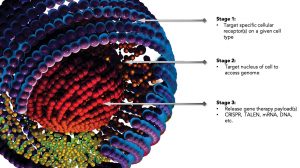
Schematic depiction of Ligandal’s gene/
protein delivery nanotechnology. The nano-
particles are peptide based and are designed
to have multiple stages of biological activity.
During delivery, the fragile nucleic acids—or, in the case of Cas9 proteins, immunogenic proteins—need to be sheltered from the immunologic system until they reach their target cells. Ligandal uses ligands to form nanoparticles and package genetic materials without viruses, causing cells to “eat” (endocytose) the nanoscale packages and deliver precise genetic instructions to target cells, including T cells and hematopoietic stem cells.
The technology evolved from Watson’s undergraduate work with bioengineering and nanomaterials to deliver edited gene constructs. In this work, he developed a way to coat transcription activator-like effector nucleases with layers of packaging material: an inner layer of electrostatic peptides, a middle layer of degradable silica, and finally an outer layer of cell-penetrating or cell-targeting peptides. Ultimately, the nuclease is at the core of a layered nanoparticle.
Subsequent refinements enable ligands containing CRISPR-editing constructs, messenger RNA, short interfering RNA, DNA, and other genetic or protein components to be delivered based on specific receptors on the surface of targeted cells. The targeting mechanism can be fine-tuned for specific cell types, tissues, or organs, thus minimizing side effects.
“If we can identify the uniqueness of a cell surface proteome, we can develop a computational model to simulate how our peptide ligands fit to the cell surface receptors,” Watson asserts. Then Ligandal can track the targeting molecules and/or genes that are being delivered, and it can design a delivery system to target the nucleus and to trigger either immediate or extended release.
“We ‘brute force’ thousands of formulations to identify the top hits that correspond to what we want to deliver,” he declares.
The first part of the delivery system relies on a robotics platform that synthesizes peptides in about one hour. “It’s predictive, so we don’t have to screen 1021 sequences to identify the top candidates,” Watson explains. “The body doesn’t like random sequences, so we build our peptides based on the native proteins already familiar to the body.”
Part two of the process creates nanoparticles using a water-phase electrostatic approach. “No purification is necessary,” he points out. “The ligands self-assemble around the given payloads we’d like to deliver, so we can use fluid handling robotics to develop and screen peptide nanoparticles for size, charge, and downstream biological properties. Cellular assays identify gene sequences, cell survival, payload presence, etc.”
Because Ligandal’s materials are derived from native proteins and program for both cellular and subcellular specificity, the nanomaterials progressively evolve as subsequent screening occurs. The goal is to predictively manufacture optimized nanoparticles for given cell types, payloads, and therapeutic applications.
In preclinical studies, Ligandal’s technology has successfully targeted specific cells in the blood and bone marrow. It appears to have applications in a wide range of gene therapies. The company is in discussions with potential partners in the immunologic and hematologic spaces and expects to make a collaboration announcement this summer. “Next year, we will have substantial news,” Watson predicts.
iTOP Technology
Delivery is a specific challenge for the CRISPR/Cas9 gene editing complex, too, Marco de Boer, PhD, CEO of Ntrans Technologies, points out. His firm has developed a virus-free cell targeting method called iTOP, which uses small molecules to induce the uptake of therapeutic proteins into the target cell. It may be used for in vivo or ex vivo applications.
“iTOP-mediated delivery is based on natural cellular uptake processes,” de Boer explains. iTOP delivers the CRISPR/Cas9 complex in the form of a ribonucleoprotein (RNP) complex that forces the uptake of large “gulps” of extracellular fluid by target cells. Each gulp yields an intracellular vesicle that contains bioactive molecules. Ultimately, the vesicles release their payload into the cytoplasm to generate a therapeutic reaction.
“This has two important benefits,” de Boer points out. “The CRISPR/Cas9 complex is immediately active and efficiently introduces the desired modification. Because the complex is broken down after a relatively short time, the risk of off-target effects is reduced. In addition, delivery in the form of RNP complex avoids the immune responses possible with viral delivery.”
Additional advantages include ex vivo and in vivo delivery of gene editing systems, virus-free delivery, cost-effective therapeutic formulation, consistency, GMP compatibility, and delivery of naïve, unmodified proteins.
NTrans’ proof-of-concept data suggests iTOP may be used for in vivo, in-eye applications of CRISPR gene editing technologies. “We are focusing on corneal and retinal applications,” de Boer says.
Next, NTrans is developing a novel, proprietary CRISPR endonuclease for use with iTOP. The combination will create a new GMP-compatible platform for immunotherapy and genomic applications. “We’re taking the next steps toward first-in-man application with a particular focus on corneal and retinal applications,” de Boer declares. Eventually, he plans to apply these technologies to personalized gene editing therapies to treat cancer and genetic diseases.
Overcoming PERV for xenotransplantation
Porcine xenotransplantation research was halted in the 1990s because of the risk of transmitting porcine endogenous retrovirus (PERV) to patients during organ transplants. To help resurrect the field, genomic pioneer George Church, PhD, and researcher Luhan Yang, PhD, co-foundeed eGenesis, a company devoted to developing human-compatible organs, tissues, and cells. eGenesis is working to make porcine tissue PERV-free and as human-like as possible to enable life-saving medical interventions.
eGenesis’ development program has four key areas: cellular genome engineering to assess PERV and immunology separately, organ production, preclinical testing, and studies to enable clinical development. “We’re in the process of creating transgenic organs, engineering out PERV, and engineering in the new elements to make the organs as close to those of humans as possible,” Paul Sekhri, president and CEO of eGenesis, tells GEN.
“We’re creating three models of pigs,” Sekhri says. “The first is PERV-free.” The second incorporates genetic modifications to enhance compatibility with the human immune system. The third blends both models. To date, eGenesis has produced PERV-free and immune models.
“Every organ will carry the genetic modifications,” Yang adds, so multiple tissues can be delivered to patients. eGenesis currently is performing efficacy and safety testing on the engineered organs. “We can’t disclose the details, but have very exciting early data,” she says.
To develop genetically modified clones, “we first modify single cells, isolate them, and determine whether they carry the modifications so we can choose the perfect cells before cloning an animal,” Yang continues. “Our focus on single cell modifications is what differentiates us.”
That approach minimizes unintended consequences. And because the scientific team can watch the animals over several years, she says, “we can check the pathophysiology of the pigs to understand any long-term impacts of the modifications.”
The company is working closely with the FDA to explore the technology, its ramifications, and regulatory considerations as the clinical package is developed. Sekhri anticipates beginning clinical trials in 2022.
Utilizing plant-based genome editing tools
The company known as Plantedit is aptly named. As one might expect, Plantedit uses genome editing tools to develop plants for industrial and biopharmaceutical uses. The company’s first product, a nontransgenic high oleic soya, is commercialized for industrial users.
The company plans to apply the same technology to develop pure mammalian proteins and human drugs from plants. The goal is to use plant-based genome editing tools to significantly enhance protein or drug yields and thus lower prices.
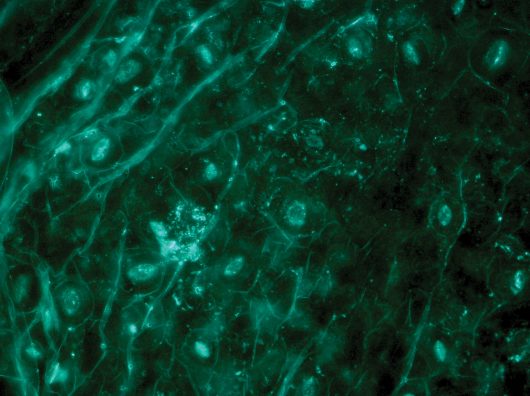
Plantedit uses CRISPR/Cas9 RNPs developed by Toolgen to produce nontransgenic, sustainable, consumer-oriented genome edited plant products. In this image, the direct delivery of genome editing tools during transfection is assessed by means of fluorescence microscopy, which shows the position of the carrying element in an Arabidopsis leaf.
Plantedit uses CRISPR/Cas9 RNPs®, tools developed by its primary collaborator, Toolgen, to achieve plant transformation. Currently, the company is working with several model plants, modulating selected genes to enhance yield and quality of the animal proteins or human drugs.
Initial work focuses on crop plants, including soy, potatoes, grapevines, and strawberries. Other commercially relevant crops will be added soon. “Our focus is to develop crops that can withstand climate change, pathogen infection, biotic or abiotic stress, and herbicides while increasing yields,” says Plantedit’s CEO, Chidananda N. Kanchiswamy, PhD. “The optimal plant model has not yet been selected.”
“We would like to bring all these economically important traits into novel germplasms that are specific to geographical regions, and directly deliver genome editing tools into the plant’s cells without introducing foreign genetic materials.”
Doing so requires delivering genome editing tools without using agrobacterium or plasmid vectors. How Plantedit accomplishes this feat is a trade secret, but Kanchiswamy states that his team can deliver the tools “without removing the cell wall and without going through regeneration hurdles.”
The benefits of using nontransgenic plants could be huge. Such plants would not be subject to the regulations that apply to genetically modified crops. Consequently, nontransgenic plants would be more acceptable to global markets.
S O U R C E : Genetic Engineering & Biotechnology News











































