.
F U L L T E X T S O U R C E : AgING
Abstract
Endogenous hydrogen sulfide mediates anti-aging benefits of dietary restriction (DR). However, it is unclear how H2S production is regulated by pathways related to DR. Due to the importance of mTORC1 pathway in DR, we investigated the effects of Sch9, a yeast homolog of mammalian S6K1 and a major substrate of mTORC1 on H2S production in yeast Saccharomyces cerevisiae. We found that inhibition of the mTORC1-Sch9 pathway by SCH9 deletion, rapamycin or myriocin treatment resulted in a dramatic decrease in H2S production. Although deficiency of SCH9 did not alter the intracellular level of methionine, the intracellular level of cysteine increased in Δsch9 cells. The expression of CYS3 and CYS4, two transsulfuration pathway genes encoding cystathionine gamma-lyase (CGL) and cystathionine beta-synthase (CBS), were also decreased under mTORC1-Sch9 inhibition. Overexpression of CYS3 or CYS4 in Δsch9 cells or WT cells treated with rapamycin rescued the deficiency of H2S production. Finally, we also observed a reduction in H2S production and lowering of both mRNA and protein levels of CGL and CBS in cultured human cells treated with rapamycin to reduce mTORC1 pathway activity. Thus, our findings reveal a probably conserved mechanism in which H2S production by the transsulfuration pathway is regulated by mTORC1-Sch9 signaling.
Introduction
The roles of Hydrogen sulfide (H2S) as a gaseous signal transmitter has been well-appreciated in the last two decades [1–4]. There are many signaling pathways in a range of organisms, from yeast to human, that are regulated by H2S including cell death, the cell cycle, autophagy, inflammation, aging and oxidative stress. Physiologically, H2S plays an important role in protecting the nervous system and the cardiovascular system of animals [5, 6].
Endogenous production of H2S is mainly catalyzed by four enzymes involved in cysteine metabolism including cystathionine gamma-lyase (CGL), cystathionine beta-synthase (CBS), cysteine aminotransferase (CAT) and 3-mercaptopyruvate sulfurtransferase (3MST). The production of H2S can be controlled by the expression of these enzymes, the bioavailability of their substrates, and enzyme activity modulating factors [3]. Therefore, the regulation of H2S production is complicated and more studies are required to clarify how it is controlled under physiological or pathological conditions.
Recently it was suggested that endogenous H2S production due to sulfide amino acids restriction is essential for anti-aging benefits of dietary restriction (DR) [7]. Similarly, methionine restriction extends eukaryotic life span probably through a mechanism involved in H2S production as well [8]. Mechanistic target of rapamycin complex 1 (mTORC1) pathway also plays a key role in the anti-aging effects of DR [9, 10]. Inhibiting mTORC1 pathway by rapamycin treatment or by deletion of down-stream signaling components such as SCH9, an homologue of mammalian S6K1 in Saccharomyces cerevisiae and one of direct substrates of mTORC1, mimics DR and provides anti-aging benefits [11–13]. However, it is unknown if the mTORC1 pathway regulates H2S production even though it mediates at least some effects of DR. Since the mTORC1-Sch9 pathway in yeasts responds to DR [14, 15] and is required for protein synthesis and amino acid metabolism [16–18], we sought to determine if mTORC1-Sch9 regulates H2S production via sulfide amino acids metabolism.
Results
Inhibiting mTORC1-Sch9 inhibits H2S production
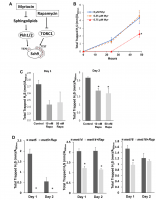
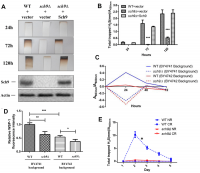
Sch9 is a direct substrate of yeast mTORC1 and depletion of SCH9 extends yeast lifespan through mechanisms shared with lifespan extension by calorie restriction (CR) [13, 16]. Since H2S mediates the benefits of CR, we first compared H2S production in Δsch9 mutant cells to WT cells. While WT cells released measurable amounts of H2S, Δsch9 cells produce barely detectable amounts of H2S (Figure 1A and 1B). H2S production was recovered if a functional SCH9 gene was added back to the mutant cells (Figure 1A and 1B), thus, showing that Sch9 activity is required for H2S production. Western blotting for Sch9 was used to verify that H2S production correlated with the concentration of Sch9 protein present in cells (lower panels, Figure 1A).
Figure 1. Deletion of SCH9 decreased H2S production in different yeast strains. (A) WT and Δsch9 cells in the TB50a background were transformed with pRS316-SCH9 or empty vector and inoculated into 1L of SDC medium at initial OD600nm=0.005. H2S production was monitored using lead acetate strips at indicated times (Upper 3 panels) after inoculation. The level of Sch9 protein and actin loading control were determined by Western blotting as shown in the lower 2 panels. (B) Millimeters of darkening of the lead acetate strips inserted into the headspace of the culture flask shown in panel A normalized by OD600nm. (C) Methylene blue assays of H2S produced by WT and Δsch9 cells in BY4741 or BY4742 background. Note that there is spontaneous oxidation of methylene blue when H2S is absent which gave negative readings for methylene reduction (red and blue dash lines). (D) Intracellular H2S production in WT and Δsch9 cells in BY4741 or BY4742 background monitored by H2S fluorescent with probe WSP-1. (* p<0.05; ** p<0.01; *** p<0.005). (E) H2S production by WT and Δsch9 cells in BY4742 background assayed by using lead acetate strips which were replaced every 24 hours under caloric restriction conditions (CR, medium containing 0.5% glucose) or no restriction (NR, medium containing 2% glucose).
Decreased H2S production by Δsch9 cells was also observed by measuring the reduction of methylene blue in different yeast strain backgrounds (BY4741 and BY4742) (Figure 1C). Measurement of intracellular H2S in these two yeast strains by using WSP-1 fluorescent showed the same trends (Figure 1D). Consistent with previous studies showing that CR enhanced H2S production in yeast [7], we observed a significant increase of H2S production in WT cell under CR (Figure 1E). However, similar to no restriction condition, H2S production was still significantly impaired in Δsch9 cell even under CR (Figure 1E).
To investigate if phosphorylation of Sch9, which correlates with its kinase activity and is required for H2S production, two inhibitors, Myriocin and Rapamycin, that indirectly lower Sch9 activity were used to inhibit the phosphorylation of Sch9 at the activation loop and the hydrophobic motif, respectively [19] (Figure 2A). Treatment with myriocin at 0.75 μM, but not 0.25 μM, inhibited H2S production (Figure 2B). Similarly, Rapamycin treatments at 10 or 50 nM also resulted in decreased H2S production (Figure 2C). These data indicate that the phosphorylation of Sch9 at the both activation loop and the hydrophobic motifs are required to the regulate H2S production.
Figure 2. Inhibiting Sch9 activity by rapamycin or myriocin treatment decreased H2S production. (A) Diagram showing how rapamycin and myriocin inhibit Sch9 through two different signaling pathways. (B) H2S production by BY4741 was monitored by using lead acetate strips at 24 or 48 hours after inoculation into YPD medium containing the indicated concentrations of myriocin. (C and D) H2S production by BY4741 or sulfur assimilatory mutants was monitored by using lead acetate strips which were replaced every 24 hours after the indicated concentrations of rapamycin were added into overnight culture of YPD (* p<0.05 compared to control).
Unlike mammalian cells, yeast cells convert extracellular sulfate to sulfide through the sulfur assimilatory pathway with enzymes encoded by MET14, MET16, and MET5/10 in addition to conserved the TSP pathway [7]. To verify if the TSP pathway is involved in decreased H2S production by mTORC1-Sch9 inhibition in yeast, we monitored the effect of rapamycin in H2S production by MET14, MET16 and MET5 mutants. As shown in Figure 2D, all three mutants have decreased H2S production upon mTORC1 inhibition by rapamycin, suggesting that interfering with the sulfur assimilatory pathway does not change the inhibitory role of rapamycin in H2S production and the TSP pathway is likely involved.
.../...
.