.
O P E N A C C E S S S O U R C E : AgING
Abstract
Rapamycin delays multiple age-related conditions and extends lifespan in organisms ranging from yeast to mice. However, the mechanisms by which rapamycin influences longevity are incompletely understood. The objective of this study was to investigate the effect of rapamycin on NAD+/NADH redox balance. We report that the NAD+/NADH ratio of C2C12 myoblasts or differentiated myotubes significantly decreases over time in culture, and that rapamycin prevents this effect. Despite lowering the NADH available to support ATP generation, rapamycin increases ATP availability, consistent with lowering energetic demand. Although rapamycin did not change the NAD+/NADH ratio or steady-state ATP concentration in the livers, kidneys, or muscles of young mice, optical redox imaging revealed that rapamycin caused a substantial decline in the NADH content and an increase in the optical redox ratio (a surrogate of NAD+/NADH redox ratio) in muscles from aged mice. Collectively, these data suggest that rapamycin favors a more oxidized NAD+/NADH ratio in aged muscle, which may influence metabolism and the activity of NAD+-dependent enzymes. This study provides new insight into the mechanisms by which rapamycin might influence the aging process to improve health and longevity among the aging population.
Introduction
It is estimated that the fraction of the global population over the age of 60 years will reach 20% in the near future, and health-care costs will rise correspondingly [1]. Thus, there is a growing recognition that solutions must be found to keep people healthy longer. Aging is a complex and multifaceted process. Nevertheless, research has demonstrated that health and longevity can be extended by calorie restriction [2], medications such as metformin [3], rapamycin [4], ibuprofen [5], resveratrol [6], spermidine [7], and supplementation of nicotinamide adenine dinucleotide (NAD+) [8], exposure to young blood [9], transfer of extracellular vesicles containing nicotinamide phosphoribosyltransferase [10], or elimination of senescent cells [11]. More recently, Fahy et al. reported that epigenetic aging can be reversed in human cells in vivo using a cocktail of drugs that was designed to promote regeneration of the thymus [12]. Collectively, these observations raise hopes that intervention in human aging may be possible.
Rapamycin and its analogs are clinically approved drugs that prevent solid organ allograft rejection and are used in the treatment of certain cancers. Rapamycin is an inhibitor of mechanistic target of rapamycin (mTOR), which has wide-ranging effects on growth and metabolism across tissues. Rapamycin extends maximal lifespan in model organisms ranging from yeast to mice, and a few studies suggest that rapamycin may also promote healthy aging in humans [4, 13, 14]. In addition to promoting survival, rapamycin is also protective in several mouse models of chronic disease [1, 15], and suppresses geroconversion which is a conversion from reversible cell cycle arrest to irreversible senescence [16]. Despite extensive studies, it remains unclear how exactly rapamycin affects longevity and diseases.
Maintaining NAD+ redox balance is necessary for optimum cellular health and may be compromised over the course of natural aging. NAD+, which is interconverted between its oxidized (NAD+) and reduced (NADH) forms, is an endogenous coenzyme and co-substrate that has key roles in diverse cellular and physiologic processes including energy metabolism and signaling through enzymes such as poly ADP-ribose polymerases (PARPs) and sirtuins. The NAD+/NADH ratio in a given cellular compartment represents the redox state, which is influenced by, and in turn regulates, metabolic activity [12]. A decrease in the NAD+/NADH ratio, reduced cellular NAD+ level, and increased NADH have all been observed during aging [17–19]. However, the effect of rapamycin on the NAD+/NADH redox state has not been thoroughly investigated.
The cell has two major routes to re-oxidize NADH to NAD+, the mitochondrial electron transport chain and lactate dehydrogenase (with subsequent export of lactate). In the absence of a functional electron transport chain, cultured cells are completely dependent on a source of exogenous oxidizing equivalents (typically pyruvate) to oxidize NADH and maintain growth [20, 21]. Under conditions of high cell density due to long-term culture or high seeding density, the release of lactic acid produced via aerobic glycolysis, which is necessary to dispose of excess reducing equivalents, acidifies the culture media. High lactate and low extracellular pH may feed back to inhibit the lactate dehydrogenase reaction, preventing cells from regenerating NAD+ from NADH and thus resulting in a reduced redox ratio, similar to that observed in aged tissues in vivo [18, 22–24]. Interestingly, rapamycin has been shown to decrease lactate production in cultured cells, raising the possibility that it can also shift NAD redox balance [25].
In the present study, we employ multiple methods, including the optical redox imaging techniques pioneered by Chance et al. [26–29], to investigate the effects of rapamycin on NAD+/NADH redox status in cultured cells, and in old and young mice in order to gain a more complete understanding of the mechanisms by which rapamycin may influence mammalian physiology.
Results
NAD+/NADH ratios were decreased in C2C12 myoblasts cultured at high density
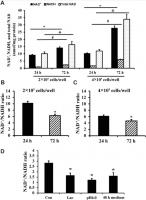
The concentrations of NAD+ and NADH and the size of the total NAD (NAD+ + NADH) pool in C2C12 myoblasts increased over time in culture (72 h compared to 24 h) (Figure 1A). Comparison of 72 h cultures at two different seeding densities showed that NAD+, NADH, and total NAD were also increased with cell density (Figure 1A). In addition, NAD+/NADH ratio of C2C12 myoblasts decreased with the extension of culture time or high seeding density (Figure 1B, 1C). A decrease in NAD+/NADH ratio was recapitulated by culturing C2C12 myoblasts under low pH, high lactate, or in conditioned medium from cells plated at high density for 48 h (Figure 1D). Thus, lactic acid buildup in the medium likely contributed to the redox shift observed over time in cell cultures.
Figure 1. NAD+/NADH ratios in long-term cultured C2C12 myoblasts. (A) NAD+, NADH, and total NAD concentrations of C2C12 myoblasts (2×105 cells/well and 4×105 cells/well) cultured for 24 and 72 h, respectively. *, P < 0.05 for comparison of NAD+ concentrations between cells cultured for 24 h and 72 h; #, P < 0.05 for comparison of NADH concentrations between cells cultured for 24 h and 72 h. &, P < 0.05 for comparison of total NAD+ concentrations between cells cultured for 24 h and 72 h. (B) NAD+/NADH ratio of C2C12 myoblasts (2×105 cells/well) cultured for 24 and 72 h; *, P < 0.05 versus cells cultured for 24 h group. © NAD+/NADH ratio of C2C12 myoblasts (4×105 cells/well) cultured for 24 and 72 h; *, P < 0.05 versus cells cultured for 24 h group. (D) NAD+/NADH ratio of C2C12 myoblasts (2×105 cells/well), which were cultured for 24 h, and then treated by lactate (10 mM), pH6 medium, and 48 h medium (collected from C2C12 myoblasts which were cultured at 4×105 cells/well for 48 h) for 24 h, respectively. *, P < 0.05 versus control group (Con, receiving no treatment). All data shown as mean ± SEM. Statistical tests were done by the Student's t test.
Rapamycin restored NAD+/NADH ratio in long-term cultured C2C12 myoblasts
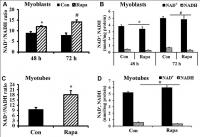
We cultured C2C12 myoblasts for 24-72 h and then subjected them to 24 h rapamycin treatment. Although the NAD+/NADH ratio of freshly plated C2C12 myoblasts (2×105 cells/well, cultured for 24 h) was not significantly changed by 24 h rapamycin treatment (data not shown), rapamycin significantly increased the NAD+/NADH ratio and decreased NADH concentration of C2C12 myoblasts cultured for either 48 h or 72 h (Figure 2A, 2B). Similarly, rapamycin significantly increased the NAD+/NADH ratio (Figure 2C) and decreased NADH concentration (Figure 2D) in C2C12 myoblasts that had been cultured longer and differentiated into myotubes (P < 0.05). Therefore, rapamycin significantly affected NADH and NAD+/NADH ratio but not NAD+ (despite an uptrend in the differentiated myotubes) in C2C12 myoblasts cultured longer than 24 h.
Figure 2. Effect of rapamycin on NAD+/NADH ratio of longer-term cultured C2C12 myoblasts and myotubes. (A) NAD+/NADH ratio of C2C12 myoblasts (2×105 cells/well) cultured for 48 h and 72 h, then were treated by rapamycin (100 nM) for 24 h, respectively. *, P < 0.05 comparing control (cultured for 48 h followed by 24 h vehicle treatment) versus cells treated by rapamycin for 24 h group by Student’s t test. # P < 0.05 comparing control (cultured for 72 h followed by 24 h vehicle treatment) versus cells treated by rapamycin for 24 h group. (B) NAD+ and NADH concentration of C2C12 myoblasts. *, P < 0.05 control versus cells cultured for 48 h and then treated by rapamycin. #, P < 0.05 control versus cells cultured for 72 h and then treated by rapamycin. © NAD+/NADH ratio of C2C12 myotubes treated by rapamycin (100 nM) for 24 h. *, P < 0.05 control versus rapamycin-treated groups. (D) NAD+ and NADH concentrations of C2C12 myotubes treated by rapamycin (100 nM) for 24 h. *, P < 0.05 versus rapamycin-treated groups.
Effect of rapamycin on ATP concentration in C2C12 myoblasts and myotubes
Since decreased lactic acid production could indicate decreased glycolysis, and we and others previously showed that rapamycin can decrease mitochondrial respiration [30], these results suggested the possibility that rapamycin might be creating an energy deficit. However, rapamycin also inhibits many energy-consuming processes, making the net effect on energy balance unclear. We observed a significant increase in ATP concentration in rapamycin-treated C2C12 myoblasts (Figure 3A) and C2C12 myotubes (Figure 3B) (P < 0.05). Thus, rapamycin has a net ATP-sparing effect, despite reducing flux through pathways of energy production.
Figure 3. Effect of rapamycin on ATP concentration in C2C12 myoblasts and myotubes. (A) ATP concentration of C2C12 myoblasts, which were cultured for 48 h, then treated by rapamycin (100 nM) for 24 h. (B) ATP concentration of C2C12 myotubes, which were cultured for 6 d, then treated by rapamycin (100 nM) for 24 h. *, P < 0.05 control versus rapamycin-treated groups by Student’s t test.
Rapamycin did not change NAD+/NADH ratio and ATP concentration in kidney, liver, and muscle tissues of young mice
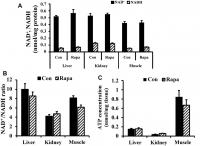
As shown in Figure 4A, 4B, rapamycin treatment did not significantly change the concentrations of NAD+ and NADH, or the NAD+/NADH ratio in kidney, liver, and muscle of young mice (2 months old). In addition, rapamycin treatment did not result in significant differences in ATP content in kidney, muscle, and liver (Figure 4C).
Figure 4. NAD redox status and bioenergetics in kidney, liver, and muscle tissues of young mice. (A) NAD+ and NADH concentrations, (B) NAD+/NADH ratio, and © ATP concentration in liver, kidney, and muscle tissues of young mice.
Rapamycin induced a more oxidized state in old mouse muscle
We also employed optical redox imaging techniques to detect rapamycin’s effects on the NAD+/NADH redox status. Optical redox imaging measures the endogenous fluorescence intensities of NADH and Fp, which represents oxidized flavoproteins containing flavin adenine dinucleotide [26–29]. The optical redox ratio Fp/(NADH + Fp) reflects the mitochondrial redox state, and there is a linear correlation between optical redox ratio Fp/(NADH + Fp) and biochemically-determined redox ratio NAD+/(NADH + NAD+) [31, 32]. Thus, the optical redox ratio can be used as a surrogate indicator of NAD+/NADH redox state. Optical redox imaging has been widely applied to metabolic studies at both the cellular and tissue level [33–35]. Compared to extraction and biochemical determination of NAD+ and NADH, optical redox imaging directly shows NADH concentration and its spatial distribution of a tissue specimen with high resolution and can be used even when tissue is very limited.
Figure 5 shows that optical redox imaging can be used to detect the redox shift induced by 24 h 100 nM rapamycin in cultured undifferentiated live C2C12 myoblasts. Figure 5A displays typical Fp, NADH, and optical redox ratio images of control and rapamycin-treated C2C12 myoblasts, showing unchanged Fp signals (indicated by similar colors), decreased NADH (indicated by dark blue color for the majority of the cells) and increased optical redox ratio on average. Quantitative analysis (n = 4) revealed that 24 h 100 nM rapamycin treatment did not significantly change Fp level, but lowered NADH level in C2C12 by 36% (P < 0.01), resulting in an uptrend of the optical redox ratio Fp/(NADH+Fp) (P = 0.09) (Figure 5B). These results are consistent with rapamycin effects on C2C12 myoblasts obtained with biochemical analysis of NADH and the redox ratio (Figure 2).
Figure 5. Optical redox imaging of live C2C12 myoblasts. (A) Typical redox images of control or rapamycin-treated cells, where the color bars in Fp or NADH images represent the intensities of the signals in arbitary unit and that for Fp/(NADH+Fp) represents the redox ratio ranging from 0 to 1; (B) Quantification of the redox imaging results (unpaired 2-tailed Student’s t test assuming unequal variance), n = 4. **, P < 0.01. All data shown as mean ± SEM.
.../...
.
Edited by Engadin, 29 September 2020 - 05:29 PM.