Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans
Abstract
Time-restricted feeding (TRF) is a form of intermittent fasting that involves having a longer daily fasting period. Preliminary studies report that TRF improves cardiometabolic health in rodents and humans. Here, we performed the first study to determine how TRF affects gene expression, circulating hormones, and diurnal patterns in cardiometabolic risk factors in humans. Eleven overweight adults participated in a 4-day randomized crossover study where they ate between 8 am and 2 pm (early TRF (eTRF)) and between 8 am and 8 pm (control schedule). Participants underwent continuous glucose monitoring, and blood was drawn to assess cardiometabolic risk factors, hormones, and gene expression in whole blood cells. Relative to the control schedule, eTRF decreased mean 24-hour glucose levels by 4 ± 1 mg/dl (p = 0.0003) and glycemic excursions by 12 ± 3 mg/dl (p = 0.001). In the morning before breakfast, eTRF increased ketones, cholesterol, and the expression of the stress response and aging gene SIRT1 and the autophagy gene LC3A (all p < 0.04), while in the evening, it tended to increase brain-derived neurotropic factor (BNDF; p = 0.10) and also increased the expression of MTOR (p = 0.007), a major nutrient-sensing protein that regulates cell growth. eTRF also altered the diurnal patterns in cortisol and the expression of several circadian clock genes (p < 0.05). eTRF improves 24-hour glucose levels, alters lipid metabolism and circadian clock gene expression, and may also increase autophagy and have anti-aging effects in humans.
1. Introduction
Intermittent fasting (IF) covers a broad class of interventions that alternate periods of eating and extended fasting. IF interventions include periodic 24-hour fasts, intermittent energy restriction (e.g., the 5:2 diet), and time-restricted feeding. In animal models, IF has been found to improve cardiometabolic health, reduce cancer incidence, slow tumor growth, regenerate organs by increasing stem cell production, and increase lifespan [
1,
2]. In humans, data on IF is limited but suggest that it decreases body weight, insulin levels, blood pressure, inflammation, and appetite, and that it improves insulin sensitivity and lipid profiles [
1,
3,
4,
5]. These clinical benefits are driven by a reduction in insulin levels; improved insulin signaling; a reduction in oxidative stress; an increase in antioxidant defenses and autophagy; a reprogramming of aging-related pathways and hormones such as sirtuin 1 (SIRT1), brain-derived neurotrophic factor (BDNF), mechanistic target of rapamycin (mTOR), and insulin-like growth factor (IGF-1); and other mechanisms [
6,
7].
While the benefits of some types of IF may stem mostly or entirely from energy restriction [
8,
9], one form of IF, called time-restricted feeding (TRF), has demonstrated benefits independent of energy restriction in both animals [
10,
11,
12,
13,
14,
15,
16,
17] and humans [
18,
19]. Since the median American eats over a 12-hour period [
20], we define TRF as eating within a ≤10-hour period and fasting for at least 14 hours per day. (Although TRF can include Ramadan fasting, we consider Ramadan fasting to be a separate type of IF.) Studies in rodents report that TRF reduces body weight, improves glycemic control, lowers insulin levels, reduces blood pressure, prevents hyperlipidemia, decreases hepatic fat, improves inflammatory markers, slows tumor growth, and increases lifespan, even when food intake is matched to the control group [
10,
11,
12,
13,
14,
15,
16,
17,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40]. To date, there have been nine pilot-sized trials of TRF in humans [
18,
19,
41,
42,
43,
44,
45,
46,
47]. Interestingly, TRF improved weight loss and cardiometabolic endpoints—such as insulin levels, insulin sensitivity, and blood pressure—when participants ate early or in the middle of the day [
18,
19,
41,
42,
43,
44,
45], but worsened cardiometabolic health or had null effects when participants ate late in the day [
46,
47,
48].
The circadian system may explain these dichotomous time-of-day effects. The circadian system orchestrates approximately 24-hour rhythms in metabolism, physiology, and behavior. It produces these rhythms through coordinated transcriptional–translational feedback loops involving clock genes such as
BMAL1, CLOCK, PER1/2, and
CRY1/2, which in turn cause oscillations in a myriad of downstream targets. For instance, insulin sensitivity and the thermic effect of food exhibit 24-hour rhythms, peaking in the morning [
49]. A large number of plasma lipids [
50] and age-related hormones such as cortisol, insulin, and growth hormone [
51,
52] also vary across the 24-hour day. Many of these metabolic and hormonal rhythms peak in the morning and are downregulated in the evening, implicating the morning as optimal for food intake [
49]. Therefore, eating in sync with these rhythms may improve cardiometabolic health, as suggested by a growing number of human studies [
53,
54,
55,
56,
57,
58]. In contrast, eating in circadian misalignment with these rhythms by eating late in the day worsens several cardiometabolic endpoints, particularly glucose tolerance [
59,
60,
61,
62]. Therefore, TRF interventions where food intake is limited to early in the day may be particularly effective at improving cardiometabolic health.
We recently conducted the first clinical trials of early time-restricted feeding (eTRF), which combines the benefits of intermittent fasting with eating early in the day to be in sync with circadian rhythms in metabolism [
18,
19]. eTRF is tantamount to eating dinner in the mid-afternoon and fasting for the rest of the day. In our first 5-week crossover study, we found that eTRF reduces insulin levels, improves insulin sensitivity, lowers blood pressure, and decreases lipid peroxidation in men with prediabetes [
18]. In our second 4-day crossover study, we investigated the effects of eTRF on energy metabolism in adults who are overweight and found that eTRF does not affect energy expenditure but increases fat oxidation, reduces the hunger hormone ghrelin, and improves subjective appetite [
18,
19]. Here, we extend our analyses from the 4-day trial to perform the first study of how TRF affects diurnal patterns in cardiometabolic risk factors, selected hormones, and the expression of glycemic and circadian clock genes in humans. As an exploratory aim, we also investigated the effects on the expression of genes related to aging, autophagy, and oxidative stress. We hypothesized that eTRF would decrease mean 24-hour glucose levels, positively impact hormones such as IGF-1 and BDNF, and alter circadian clock gene expression.
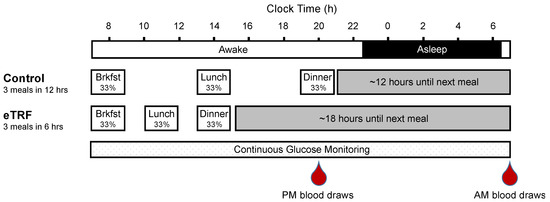
Figure 1. Study Protocol. Eleven participants were randomized to eat between 08:00 and 20:00 control arm) or between 08:00 and 14:00 (early time-restricted feeding (eTRF) arm) for 4 days and then crossed over to the other arm after a 3.5–5-week washout period. On day 4, they consumed 3 identical meals that constituted one-third of their daily energy requirements, while undergoing 24-hour continuous glucose monitoring. In addition, blood was drawn in the evening (PM) on day 3 and in the morning (AM) on day 5 to measure serum analytes and gene expression.
.....











































