S O U R C E : nature_Molecular Psychiatry
ABSTRACT
A decrease in adult hippocampal neurogenesis has been linked to age-related cognitive impairment. However, the mechanisms involved in this age-related reduction remain elusive. Glucocorticoid hormones (GC) are important regulators of neural stem/precursor cells (NSPC) proliferation. GC are released from the adrenal glands in ultradian secretory pulses that generate characteristic circadian oscillations. Here, we investigated the hypothesis that GC oscillations prevent NSPC activation and preserve a quiescent NSPC pool in the aging hippocampus. We found that hippocampal NSPC populations lacking expression of the glucocorticoid receptor (GR) decayed exponentially with age, while GR-positive populations decayed linearly and predominated in the hippocampus from middle age onwards. Importantly, GC oscillations controlled NSPC activation and GR knockdown reactivated NSPC proliferation in aged mice. When modeled in primary hippocampal NSPC cultures, GC oscillations control cell cycle progression and induce specific genome-wide DNA methylation profiles. GC oscillations induced lasting changes in the methylation state of a group of gene promoters associated with cell cycle regulation and the canonical Wnt signaling pathway. Finally, in a mouse model of accelerated aging, we show that disruption of GC oscillations induces lasting changes in dendritic complexity, spine numbers and morphology of newborn granule neurons. Together, these results indicate that GC oscillations preserve a population of GR-expressing NSPC during aging, preventing their activation possibly by epigenetic programming through methylation of specific gene promoters. Our observations suggest a novel mechanism mediated by GC that controls NSPC proliferation and preserves a dormant NSPC pool, possibly contributing to a neuroplasticity reserve in the aging brain.
INTRODUCTION
Aging imposes an increasing disease burden and the neurological consequences of aging, such as cognitive decline, are particularly deleterious to quality of life [1]. There is substantial heterogeneity in the various changes in brain function associated with aging, suggesting that aging proceeds at different rates due to genetic, environmental, emotional and/or physiopathological factors [2]. Among the latter, alterations in circadian glucocorticoid hormones (GC) rhythms are associated with increased allostatic load and may affect normal aging [3,4,5]. GC are rhythmically released from the adrenal glands in ultradian near-hourly pulses. These ultradian pulses generate characteristic circadian oscillations in circulating GC levels [6, 7]. GC oscillations develop after the third week of life in mice [8] and induce cyclic glucocorticoid receptor (GR)‐mediated transcriptional regulation, or gene pulsing, in vitro [9] and also in vivo in the hippocampus [10]. Alterations in GC oscillations are observed in aged mammals, including mice [11] and humans [6]. GC oscillations have been implicated in the regulation of cortical plasticity [12], anxiety-like behavior [13], and the diurnal rhythm of neural stem/precursor cells (NSPC) proliferation in the dentate gyrus (DG) [14].
NSPC in the sub-GZ (SGZ) of the DG proliferate and generate new neurons in the adult hippocampus across the lifespan of most mammals [15,16,17,18,19,20,21]. Several studies have documented an age-associated decline in NSPC proliferation, suggesting an age-dependent exhaustion of the NSPC pool [19, 22,23,24,25,26,27,28,29]. As adult NSPC proliferation may be limited to a finite number of divisions [27], NSPC quiescence could preserve a NSPC pool that contributes to neuroplasticity reserve and preservation of hippocampus-dependent cognitive functions during aging [19, 30,31,32,33]. However, this hypothesis remains controversial and subject to debate [34,35,36,37]. In particular, the underlying molecular mechanisms involved are still unknown and require detailed characterization.
NSPC dynamically and selectively respond to GC, which strongly inhibit NSPC proliferation [23, 38,39,40]. In mice, GC acting through the GR have direct effects on NSPC differentiation and functional integration within hippocampal circuits [41]. In old rats, adrenalectomy (ADX) increases NSPC proliferation in the hippocampus, whereas lifelong GC reduction increases AHN and prevents age-related memory disorders [23, 39, 42]. Interestingly, ADX induces a cellular phenotype in the DG that is very similar to the one induced by GR knockdown, i.e., a significant increase in the number of DCX+ cells and immature neurons with an ectopic location and multiple primary dendrites, indicating that the GR is of critical importance in the regulation of newborn neuron maturation [41]. However, ADX is a surgical strategy that will affect all GC-responsive cell types and remove several other adrenal hormones as well, making the identification of a direct link to cell-type specific effects impossible. The effects of GC on adult hippocampal neurogenesis (AHN) are age-dependent, as life-long GC suppression from early life onwards does not enhance AHN [43]. Therefore, the relationship between GC, NSPC proliferation and AHN is complex and remains incompletely characterized. Importantly, in young adult mice, NSPC populations exhibit differences in GR expression and response to GC stimulation [41, 44, 45].
Here, we show for the first time that GC oscillations are associated with the preservation of GR-expressing NSPC populations in the aging DG, suggesting a novel mechanism that controls the maintenance of NSPC in the aging brain and presenting a possible source of neuroplasticity reserve that could be exploited to sustain hippocampus-dependent cognitive functions throughout life.
RESULTS
GR+ NSPC populations persist into old age and decay with different kinetics in vivo
NSPC were classified based on the expression of Nestin-GFP and GFAP [16, 46, 47]. Specifically, Nestin-GFP+/GFAP+ with characteristic radial glia-like morphology were classified as Type-1 cells. Type-2a cells were Nestin-GFP+/GFAP+, with horizontal morphology and Type-2b cells were Nestin-GFP+/GFAP−, also with horizontal morphology. Type-1, -2a and -2b cells were observed in animals of all ages (Fig. S1C–I). The numbers of proliferative NSPC decreased with age in Nestin-GFP mice [27] (Fig. S1 A, B). Furthermore, extra-sum-of-squares F-testing for best-fit decay curves showed that the total Nestin-GFP+ NSPC population decayed exponentially during aging (Fig. S1J). Importantly Nestin-GFP expression was consistent with native Nestin expression over time and was unaffected by aging in individual Type 1 NSPC [27] (Fig. S2A–C). Interestingly, Type-1, -2a, and -2b cells decayed following different patterns. Type-1 and -2a cells decayed linearly, while Type-2b cells followed exponential decay kinetics (Fig. S1K). The volume of the granule zone (SGZ plus granule cell layer (GCL)) did not change significantly with age (Fig. S1J). These data demonstrate that Type-1 and -2a NSPC persist into old age, while Type-2b cells are depleted earlier following an exponential decay.
We next characterized GR expression in Type-1, -2a, and -2b cells in 3- to 18- month-old Nestin-GFP mice (Fig. 1a–q, Fig. S1L, Fig. S2D). The relative abundances of GR+ and GR− populations of Type-1, -2a, and -2b cells changed with age (Fig. S1L), in agreement with previous studies showing heterogeneous GR expression in NSPC populations in young animals [41, 44, 48]. At 3 months of age, most Type-1 and -2a cells were GR+, whereas the majority of Type-2b cells were GR- at this age. However, from 6 months of age on, GR+ cells predominated in all NSPC populations. This predominance of GR+ NSPC populations persisted throughout middle and into old age (Fig. S1L). Thus, a marked depletion of GR− NSPC takes place in DG earlier than anticipated from previous studies [44]. Interestingly the decay of GR− NSPC populations fitted best to an exponential decay, while the decay of GR+ populations fitted best to a linear model (Fig. 1r–t, Fig. S2F–K).
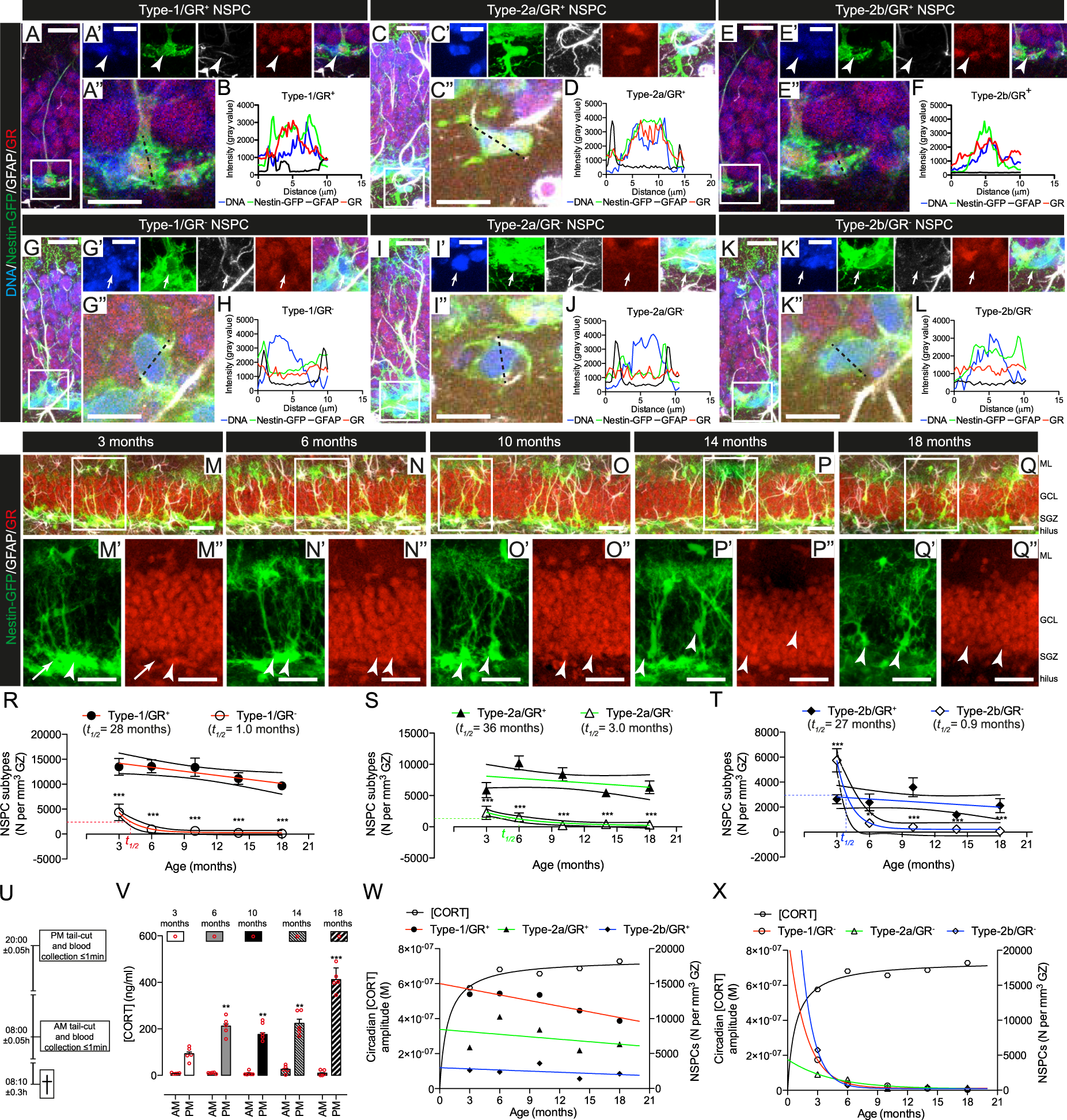
The preservation of NSPC populations is associated with GR expression and age-related changes in the amplitude of circadian CORT oscillations. a Representative example of Nestin-GFP+/GFAP+/GR+ NSPC with characteristic vertical process and triangular cell-body in the SGZ of the DG. a’ The boxed area in A is magnified and channels split and Z-stacked, showing the expression of individual markers. Arrowhead: cell soma. a” The dashed black line shows a transversal cell section. b Histogram of the transversal section in (a”), showing fluorescent intensity signals for DNA (blue), GFP (green), GFAP (black) and GR (red). Representative examples of c–d Type-2a/GR+, e–f Type-2b/GR+, g–h Type-1/GR−, i–j Type-2a/GR−, and k–l Type-2b/GR− NSPC. In all cases cells with intensity value ≥1500 across the nucleus were considered GR+(Fig. S2D). NSPC in the DG of m 3, n 6, o 10, p 14, or q 18-month-old mice. The boxed areas are shown magnified in the panels below each image. Arrows: Nestin-GFP+/GR− Type-1 NSPC; arrowheads: Nestin-GFP+/GR+ Type-1 NSPC. Scale bars represent 40 μm (m–q”); 20 μm (a, c, e, g, i, and k); 15 μm (a’, c’, e’, g’, i’, and k’) and 10 μm (a”, c”, e”, g”, i”, and k”). r Best-fit curves and 95% confidence intervals of Type-1 GR+ (solid circles) GR− (open circles); s Type-2a GR+ (solid triangles) and GR−(open triangles) or (t) Type-2b GR+ (solid diamonds) and GR− (open diamonds) cell numbers. Data points indicated by the different shapes are mean ± SEM (n = 5 mice, *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA) and NSPC population half-lives (t1/2) are indicated in the figures. GR− populations fitted exponential decay curves (p < 0.05, F-test, calculated t1/2 = 1.02 (Type-1), 3.0 (Type-2a), and 0.9 months (Type-2b) NSPC, respectively). GR+ populations fitted linear decay curves (p < 0.05, F-test, calculated t1/2 = 28 (Type-1), 36 (Type-2a) and 27 months (Type-2b) NSPC, respectively). Best curve fit comparisons are shown in Figure S2F-K. u Time-windows of blood collection. v AM and PM plasma [CORT] at different ages in mice. Bars are mean ± SEM and red circles individual data points (animals) (n = 5 mice, *p < 0.05, **p < 0.01, ***p < 0.001, vs. 3-month-old, one-way ANOVA). Calculated circadian CORT amplitude (black line) vs. w GR+ or x GR− Type-1 (red lines), -2a (green lines) and -2b (blue lines) NSPC numbers at different ages in mice
..../....









































