
By Vince Giuliano
This blog entry continues the discussion of the previous blog entry, Part 7 of the Inflammation series. So you might want to read that Part 7 before this one if you have not already done so. I continue element-by-element.
ELEMENT E: REVIEW OF SCIENTIFIC ASPECTS RELATED TO NEUROINFLAMMATION: REACTIVE OXYGEN SPECIES, MITOCHONDRIAL FACTORS, INFLAMMASOMES, PPARs, METBOLIC PATHWAYS, SIRTs, DNA DAMAGE AND ROLES OF NRF2 AND NF-KB, AND ANOTHER MAGIC MUSHROOM, YUN-ZHI

Yun Zhi (Turkey Tail) mushrooms Image source
About neuroinflammation
Like so many phenomena in biology, neuroinflammation has both good and bad sides. The good sides are essential for human life, The bad sides come with certain disease processes and aging. This duality is outlined in the 2016 publication Neuroinflammation: The Devil is in the Details. “There is significant interest in understanding inflammatory responses within the brain and spinal cord. Inflammatory responses that are centralized within the brain and spinal cord are generally referred to as “neuroinflammatory”. Aspects of neuroinflammation vary within the context of disease, injury, infection or stress. The context, course, and duration of these inflammatory responses are all critical aspects in the understanding of these processes and their corresponding physiological, biochemical and behavioral consequences. Microglia, innate immune cells of the central nervous system (CNS), play key roles in mediating these neuroinflammatory responses. Because the connotation of neuroinflammation is inherently negative and maladaptive, the majority of research focus is on the pathological aspects of neuroinflammation. There are, however, several degrees of neuroinflammatory responses, some of which are positive. In many circumstances including CNS injury, there is a balance of inflammatory and intrinsic repair processes that influences functional recovery. In addition, there are several other examples where communication between the brain and immune system involves neuroinflammatory processes that are beneficial and adaptive. The purpose of this review is to distinguish different variations of neuroinflammation in a context-specific manner and detail both positive and negative aspects of neuroinflammatory processes.”
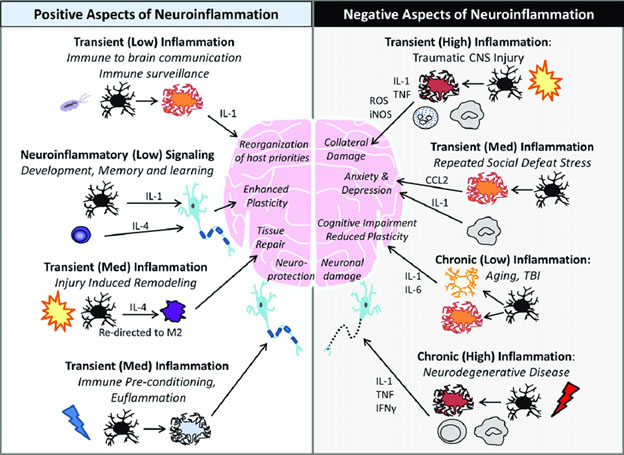
Image source: Neuroinflammation: The Devil is in the Details
Chronic neuroinflammation
Comprehending the negative aspects of chronic neuroinflammation and effectively dealing with them leads us to considering many aspect of the longevity sciences treated in the history of this blog. The best article I have seen bringing these matters together with respect to neuroinflammation is the 2017 article Neuroinflammation and Mitochondrial Dysfunction in the Pathogenesis of Alzheimer’s Disease: Modulation by Coriolus Versicolor (Yun-Zhi) Nutritional Mushrooms. I list some of the highlights from this article here, which is about much more than Alzheimer’s disease and Yun Zhi (Turkey Tail) mushrooms. But if you really want to understand these matters fit together, I suggest you read this article; then keep re-reading it reading its references until you really understand it.
I believe many if not most remarks here related to neuroinflammation regarding Alzheimers Disease are in fact applicable to all neurodegenerative diseases.: Parkinson’s Disease, ALS, and others. All are characterized by high levels of neuroinflammation.
The abstract of the article reads “Abnormal redox homeostasis and oxidative stress have been proposed to play a role in the etiology of several neuropsychiatric disorders and emerging interest has recently focused on markers of oxidative stress and neuroinflammation in neurodegenerative disorders as well as in different forms of chronic mental illness. Oxidative stress and altered antioxidant systems have been considered an important factor underlying the pathogenesis of Alzheimer’s disease (AD). Altered expression of genes related to oxidative stress, oxidative damage to DNA, protein and lipids, as well as alterations in the redox state in central and peripheral tissues could act synergistically, and contribute to the course of the disease. Specifically, we discuss the emerging role of lipoxinA4 and inflammasome in neurodegeneration. However, the notion that low levels of stress can induce responses that may be protective against the pathogenic processes is a frontier area of neurobiological research focal to understanding and developing therapeutic approaches to neurodegenerative disorders. Herein, we discuss the potential therapeutic role of Coriolus versicolor, a mushrooom, well known in China as Yun Zhi. We propose a potentially innovative treatment for AD and, possibly, other neurodegenerative conditions associated to neuroinflammation.”
Some of the important points are:
· “Brain inflammation has been linked to many of these diseases, including amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), Parkinson’s disease (PD) and, particularly, Alzheimer’s disease (AD)5.” Yes my strong intuitive hunches are a) mitigating brain inflammation may be the best approach to controlling or even curing these diseases, b) if we can do this with one of these disease, we can also probably do it with all of them. And c) we are already on our way now of being able to do this.
· “To adapt to environmental changes and survive different types of injuries, brain cells have evolved networks of responses that detect and control diverse forms of stress6,7. Consistent with this notion, integrated survival responses exist in the brain, which are under control of redox-dependent genes, called vitagenes (Figure 1), including heat shock proteins (Hsps), sirtuins, thioredoxin and lipoxin A4.” To the vitagene list I would add heme oxygenase (HO-1) which is induced by NRF-2 expresssion.
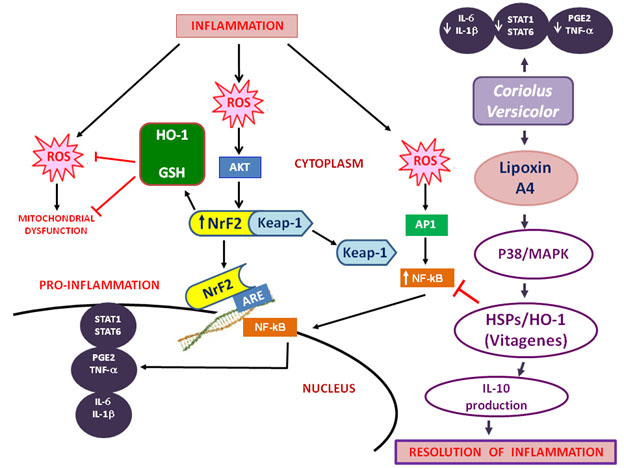
You will recognize many topics illustrated here from earlier blogs. As explained in Part 7 of the inflammation series, I think you could swap mushrooms, that is substitute Hericium Erinaceus for Coriolus Versicore in the above diagram and have the diagram be still valid. That is Lipoxin A4 seems to be the key mediator of inflammation for both mushrooms. Of course, the popular mushroom name for Hericium Erinaceus is Lion’s Mane. And, the popular mushroom name for Coriolus Versicore is Turkey Tail. “These proteins actively operate in detecting and controlling diverse forms of stress and neuronal injuries7-9. LXA4, a metabolic product of arachidonic acid, is considered an endogenous ‘‘stop signal’’ for inflammation, and shows potent anti-inflammatory properties in many inflammatory disorders, such as nephritis, periodontitis, arthritis, inflammatory bowel disease10.” The Lipoxin A4 may be very important. But other lipoxins, maresins, protectins and resolvins are also important for seeing to the resolution phase of inflammation. See the blog entry Inflammation Part 3: resolving inflammation – resolvins, protectins, maresins and lipoxins
· “The mitochondrial free radical theory of aging postulates that the damage caused by accumulating ROS produced by mitochondria is the driving force behind aging26. This theory is corroborated to some extent by the inverse correlation between mitochondrial ROS production and lifespan in mammals27. Current evidence points to mitochondrial dysfunction as an overarching mechanism of aging and age-related disease. It is implicated in an extensive list of aging pathologies such as cancer, intestinal barrier dysfunction, depression, chronic obstructive pulmonary disease (COPD), diabetes, and others28-30.” Yes I agree what goes on in mitochondria provides a special view on inflammation, but is very important. And there are several critical relationships between mitochondrial, cytosolic, and nuclear events that drive health or lack of it. Just one simplified example is that if mitochondria are damaged and give off too much ROS into the cytosol and then into the nucleus, this will result in DNA damage, causing the DNA repair machinery to kick into gear. This machinery has a priority for consumption of SIRTs. This means fewer of the sirtuins will be available for production of critical mitochondrial proteins. Starved of such proteins the mitochondria will function less well and give off even more ROS. A vicious negative cycle can thus ensue.
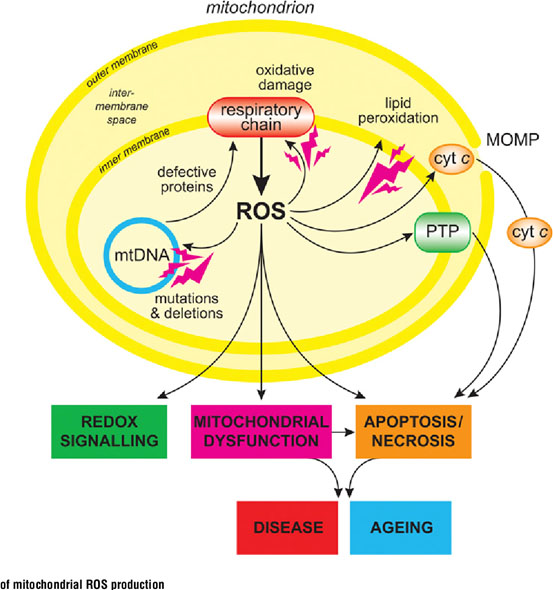
·
Image source
“ROS are not the only aspect of flawed mitochondria that contributes to degenerating health, the emerging picture is that mitochondrial dysfunction in human aging and aging-associated diseases are not limited to accumulation of mtDNA mutations, but extend to abnormalities in mitochondria biogenesis, turnover, dynamics, and proteostasis. Mitochondria undergo constant fusion and fission to maintain a balance between mitochondrial biogenesis and mitochondrial autophagy (mitophagy) or apoptosis31.” Absolutely right. More on mitochondrial biogenesis to follow here. Another key factor is mitochondrial transportation within nerve cells using kinesin and dynesin motor proteins. Nerve cells can be amazingly long and the nerve cells need mitochondria to be positioned where they are needed for energy production, See Transporting mitochondria in neurons. Nerve cell’s internal axonal railway systems breaking down can be part of the neurodegenerative picture(ref).
· “The nuclear protein, peroxisome proliferator-activated receptor-γ co-activator-1α (PGC-1α), is a key mediator of mitochondrial biogenesis and an inducer of Mfn2. Perturbations of the IMM and OMM during ROS leakage are exacerbated by disrupted fusion and fission regulatory pathways. Thus, a deleterious feedback loop between ROS and dynamics leads to mitochondrial ROS dysfunction and subsequent apoptosis. Mitochondrial Sirtuins are modulators of energy metabolism, DNA repair and oxidative stress. Sirt-1 is both a nuclear and cytoplasmic protein and has been observed in mitochondria, while Sirt-3, 4, and 5 are mitochondrial proteins33.” Yes, relevant to the example I cited above. See the blog entry Mitochondria Part 2: Mitochondrial Responses to Stress: Mitochondrial Signaling: Survival and Death Pathways.
· “Recent studies have demonstrated that the inflammasome modulates neuroinflammatory processes at the initial stage, with a secondary cascade of events inclusive of oxidative stress, having been shown to possess the ability to activate the inflammasome60. — AIM2 is a cytoplasmic sensor that recognizes dsDNA of microbial or host origin. Upon binding to DNA, AIM2 assembles inflammasomal multiprotein complex, which induces pyroptosis and proteolytic cleavage of the proinflammatory cytokines pro-IL-1β and pro-IL-18. Release of microbial DNA into the cytoplasm during infections activates the AIM2 inflammasome. For instance, inappropriate recognition of cytoplasmic self-DNA by AIM2 contributes to the development of a number of autoimmune and inflammatory diseases, as well as in neurodegenerative disorders63,64.” Yes, inflammasomes are responsible for perpetuating inflammatory processes. To learn about them see the blog entry Inflammation Part 5: Inflammasomes – science of and disease implications.
So in this article we have links between to neroinflammation and many of our old friends we have talked so much about in this blog like the mitochondria, the exercise protein PGC-1α, roles of ROS, the sirtuins, the NAD world and inflammasomes.
The 2018 article Neuroinflammation and neurohormesis in the pathogenesis of Alzheimer’s disease and Alzheimer-linked pathologies: modulation by nutritional mushrooms picks up on the same themes. This article reinforces and expands on the contents of the previous one and contains much more information on the bioactive properties of Hericium erinaceus. “ A growing number of studies have demonstrated that dietary interventions regulate mitochondrial ROS production, detoxification and oxidative damage repair. Many (but not all) of these nutritional interventions are related with extension of lifespan, or protection against diseases related with age, in mammals. Emerging nutraceuticals are today showing promise as modulators of mitochondrial redox metabolism capable of eliciting beneficial outcomes. Mushrooms, known for their strong antioxidant properties, have attracted interest due to their potential in neuroprotection, antioxidant, and anti-inflammatory effects, as well as in proteome and mitochondrial homeostasis restoration as a basic mechanism to withstand mitochondrial dysfunction-associated neuroinflammatory disorders.”
ELEMENT F NEURAL REGENERATION
I believe an important facet of this overall story is endogenous mechanisms of neural and nervous system regeneration via the Yamanaka OSKM factors. I am picking up here on the theme of my earlier blog article this year AGING, CELL AND TISSUE REPAIR, RENEWAL AND REGENERATION, INFLAMMATION AND THE SASP. ‘We are entering an era where organ repair, renewal and regeneration has been demonstrated in laboratory fish and small animals, where we are understanding the molecular mechanisms through which these can take place, and we know how to trigger them.”
In the early days after discovery of the Yamanaka factors and learning about how the OSKM factors could be used to regress just about any somatic cell into pluripotent stem-cell like status, investigators naively thought that regeneration of somatic tissue cells would be a relatively simple matter: a. we would collect some skin cells from an individual, b. then we would regress these cells back to pluripotent status using the OSKM factors, and c. we would introduce those cells back into the tissues of that person where we wanted to induce regeneration, be this in any organ, including in the brain or elsewhere in the CNS. D. There, we thought, the pluripotent cells would act like natural stem cells. Because the cells where originated in the individual in the first place, there would be no host-graft allergic reaction. This approach did not work and sometimes led to teratomas, cancer-like cysts of mixed body tissues growing around the re-injection site.
The approach was wrong in several central respects: 1. Regression back to full pluripotent status carried the cells back epigenetically too far. When you introduced pluripotent cells into a place of the body, they did have proper signaling to tell them what kind of tissues to become, so they often became several kinds mixed together. Thus, the teratomas. If we want to regenerate nerve cells, it is better to start out with somatic cells of the nerve cell lineage, and regress them only to some earlier epigenetic status in which they retain their lineage information. And 2, The OSKM regression and re-differentiation can best take place in the body right in the place where you want the regenerated nerve tissue, that is where the proper niche signaling conditions can exist for the re-differentiation to best take place. It is also where there is signaling indicating which kinds of cells of the lineage are needed (e.g. for the nerve cell lineage, neurons, oligodendrocytes, astrocytes and micro-glia) and how they can differentiate in a way to re-integrate with and renew existing tissues.
The big breakthrough for me about all of this was a little more than a year ago when I learned that for several kinds of tissue (including nervous tissues): a. such OSKM tissue renewal goes on in the body all the time, and b. that that regeneration is prompted by and requires cell-senescence signaling for its triggering.
Here I want to get back to the specific topic of neural regeneration such as discussed the 2016 publication Direct reprogramming of somatic cells into neural stem cells or neurons for neurological disorders. By 2016 we were beginning to identify specific roles of the individual Yamanaka factors, SOX2 in particular, identify how neural cells of different types can trans-differentiate (e.g. epigenetically regressed glial cells re-differentiate into neurons), and begin understanding factors relating to in-vivo renewal of neural tissues. “The ultimate goal of cell reprogramming is to seek an innovative approach for cell therapy in human diseases. With understanding the property of induced cells in vitro and establishing transgenic reporter system, it is important to determine if the conversion is applicable in vivo. Recent studies transfected human fibroblasts and astrocytes with lentiviral tet-on system expressing regulatable neural reprogramming genes (BAM). After transplanted into the rat brain, these non-neuronal cells transdifferentiated into neurons when transgene expressions were turned on by doxycycline administration (Torper et al., 2013). Also, the same study reprogramed endogenous astrocytes into neurons using the same factors. This is the first study showing that direct neuronal conversion in vivo is achievable. — Glia in the central nervous system tissue can be directly converted to neurons. It was demonstrated that a single transcription factor, Sox2 alone or together with Ascl1, converted NG2+ glia into induced doublecortin (DCX)+ neurons in the adult mouse cerebral cortex following brain injury (Heinrich et al., 2014). However, this did not happen in unlesioned cortex, indicating the necessity of signal from injury. Similarly, over-expression of a single transcription factor, Sox2, in the injured adult spinal cord, directly transformed resident astrocytes into DCX+ neuroblasts (Su et al., 2014). Another single transcription factor, NeuroD1, is also able to convert reactive glial cells into functional neurons in the cortex of stab-injured or Alzheimer’s disease mouse models (Guo et al., 2014). In this study, astrocytes were transdifferentiated into glutaminergic neurons whereas NG2+ cells were reprogrammed into glutaminergic or GABAergic neurons. Besides residential glia in the central nervous system, cochlear non-sensory epithelial cells can be re-programmed into functional neurons using Ascl1 alone or Ascl1 and NeuroD1, which may be a good indicator for the regeneration of auditory neurons in the mammalian cochlea (Nishimura et al., 2014).” Personally, I am impatiently awaiting for a practical approach for the regeneration of my auditory neurons. So I can put my hearing aids in a drawer and gradually forget that I once needed them. My guess is that this may come to be in the next 3-5 years.
In the last three years, progress has been accelerating. Specifically, as examples:
The road to understanding how effectively to induce in-situ neurogenesis when therapists think it is needed has been a rocky one. The 2017 publication Methods of reactivation and reprogramming of neural stem cells for neural repair reports “Research on the biology of adult neural stem cells (NSCs) and induced NSCs (iNSCs), as well as NSC-based therapies for diseases in central nervous system (CNS) has started to generate the expectation that these cells may be used for treatments in CNS injuries or disorders. Recent technological progresses in both NSCs themselves and their derivatives have brought us closer to therapeutic applications. Adult neurogenesis presents in particular regions in mammal brain, known as neurogenic niches such as the dental gyrus (DG) in hippocampus and the subventricular zone (SVZ), within which adult NSCs usually stay for long periods out of the cell cycle, in G0. The reactivation of quiescent adult NSCs needs orchestrated interactions between the extrinsic stimulis from niches and the intrinsic factors involving transcription factors (TFs), signaling pathway, epigenetics, and metabolism to start an intracellular regulatory program, which promotes the quiescent NSCs exit G0 and reenter cell cycle. Extrinsic and intrinsic mechanisms that regulate adult NSCs are interconnected and feedback on one another. Since endogenous neurogenesis only happens in restricted regions and steadily fails with disease advances, interest has evolved to apply the iNSCs converted from somatic cells to treat CNS disorders, as is also promising and preferable. To overcome the limitation of viral-based reprogramming of iNSCs, bioactive small molecules (SM) have been explored to enhance the efficiency of iNSC reprogramming or even replace TFs, making the iNSCs more amenable to clinical application. Despite intense research efforts to translate the studies of adult and induced NSCs from the bench to bedside, vital troubles remain at several steps in these processes. In this review, we examine the present status, advancement, pitfalls, and potential of the two types of NSC technologies, focusing on each aspects of reactivation of quiescent adult NSC and reprogramming of iNSC from somatic cells, as well as on progresses in cell-based regenerative strategies for neural repair and criteria for successful therapeutic applications.”
The 2018 publication Cell Signaling in Neuronal Stem Cells deals with the complex mechanisms involved in neurogenesis. It reports “— The process by which new neurons are generated is called neurogenesis; this involves multiple and complex pathways [3]. The NSCs give rise through asymmetric cell divisions, to the neural precursor cells which in turn by this same type of cell division, give rise to new functional neurons, both in the embryonic neural development and in the adult CNS. This creation of a new functional neuron includes the self-renewal of neural stem cells and neural precursor cells, the generation of neuroblasts that differentiate into young neurons that migrate, mature, and integrate into the pre-existing neuronal circuit, processes regulated by the dynamic interaction between the genome, epigenetic mechanisms, and extrinsic signals (Figure 1) [4].” — The management involving stem cell (SC) therapy along with physiotherapy offers tremendous chance for patients after spinal cord injury (SCI), traumatic brain injury (TBI), stroke, etc. However, there are still only a limited number of reports assessing the impact of stem cells (SCs) on the rehabilitation process and/or the results of the simultaneous use of SC and rehabilitation. Additionally, since there is still not enough convincing evidence about the effect of SCT on humans, e.g., in stroke, there have been no studies conducted concerning rehabilitation program formation and expected outcomes. It has been shown that bone marrow-derived mesenchymal stem cell (BMSCs) transplantation in rats combined with hyperbaric oxygen therapy (HBO) can promote the functional recovery of hind limbs after SCI. An anti-inflammatory effect has been shown. One case study showed that, after the simultaneous use of SCT and rehabilitation, an SCI patient progressed from ASIA Grade A to ASIA Grade C. Such promising data in the case of complete tetraplegia could be a breakthrough in the treatment of neurologic disorders in humans. Although SCT appears as a promising method for the treatment of neurological conditions, e.g., complete tetraplegia, much work should be done towards the development of rehabilitation protocols.”
Doxycycline, a neurogenerative longevity drug?
The drug Doxycycline keeps coming up in the scientific literature associated with longevity, but never in a way that had particularly grabbed my attention. Most of the references seem to have been to uses of the Tet-on Tet-off system whereby the drug can be used as a switch to activate transcription to turn genes off and on. A week ago I was prescribed a 21-day course of doxycycline for a sinus and minor prostate condition, so I decided to pay a little attention to what is known about the drug. Being in the middle of the course of taking the drug I was amazed with what I found yesterday, as outlined here.
Doxycyline Image source
First of all, “Doxycycline works by inhibiting bacterial protein synthesis by binding to a ribosomal subunit, preventing amino acids from being linked together. Without proteins, bacteria are unable to function(ref).” By this mechanism, doxycycline also inhibits certain forms of normal human protein synthesis, creating many actions in addition to slowing or stopping reproduction of unwanted bacteria. Doxycycline is a synthetic antibiotic of the tetracycline inhibitors group. Its reported effects are on pain, inflammation and neuroprotection (Clark et al., 1997; Cho et al., 2009; Jantzie and Todd, 2010; Yoon et al., 2012)(ref).”
Let’s next consider the 2013 publication Doxycycline increases neurogenesis and reduces microglia in the adult hippocampus. “Adult hippocampal neurogenesis results in the continuous formation of new neurons and is a process of brain plasticity involved in learning and memory. Although inducible-reversible transgenic mouse models are increasingly being used to investigate adult neurogenesis, transgene control requires the administration of an activator, doxycycline (Dox), with unknown effects on adult neurogenesis. Here, we tested the effect of Dox administration on adult neurogenesis in vivo. We found that 4 weeks of Dox treatment at doses commonly used for gene expression control, resulted in increased neurogenesis. Furthermore, the dendrites of new neurons displayed increased spine density. Concomitantly, Iba1-expressing microglia was reduced by Dox treatment. — The mechanisms regulating adult neurogenesis are highly relevant for our understanding of brain plasticity and for the potential use of these cells as therapeutic targets. Indeed, although their role remains unclear, increasing evidence suggests that new neurons are involved in mechanisms of learning and memory (Van Praag et al., 1999, 2002; Saxe et al., 2006, 2007; Dupret et al., 2007; Trouche et al., 2009; Massa et al., 2011; Gu et al., 2012; Shors et al., 2012; Tronel et al., 2012) as well as in depression and mood control (Santarelli et al., 2003; Samuels and Hen, 2011).” OK, The “adults” in the article title are adult mice. But because our relevant pathways are so similar, there is a good chance doxycycline upgrades neurogenesis in us as well.
Neurogenesis is also key for averting or treating neural diseases(ref)(ref).
A 2019 publication directly relevant to the subject of this blog entry is The Neuroprotective Effect of Doxycycline on Neurodegenerative Diseases. It suggests that doxycycline is not only interesting for working with animal disease models, but it might be of direct relevance for treatment or prevention of human neuroinflammatory diseases. “Neurodegenerative diseases (NDs) are a group of chronic, progressive disorders characterized by the gradual loss of neurons that affect specific regions of the brain, which leads to deficits in specific functions (e.g., memory, movement, cognition). The mechanism that drives chronic progression of NDs remains elusive. Among the proposed underlying pathophysiological mechanisms, aggregation and accumulation of misfolded proteins and neuroinflammation have been credited to contribute to extensive neural loss. Therapeutic agents that confer neuroprotection by downregulating these shared characteristics could therefore have beneficial effects on a wide range of NDs. In this regard, a commonly used antibiotic, doxycycline (Doxy), has been shown to reduce the progression and severity of disease in different experimental models of neurodegeneration by counteracting these common features. This review will focus on the effects reported for Doxy regarding its neuroprotective properties, the “off-target” effects, thereby supporting its evaluation as a new therapeutic approach for diseases associated with a neurodegeneration.”
Another highly relevant 2019 article is Doxycycline for Alzheimer’s Disease: Fighting β-Amyloid Oligomers and Neuroinflammation. “Alzheimer’s disease (AD) is the most widespread form of dementia, affecting about 45 million people worldwide. Although the β-amyloid peptide (Aβ) remains the most acknowledged culprit of AD, the multiple failures of Aβ-centric therapies call for alternative therapeutic approaches. Conceivably, the complexity of the AD neuropathological scenario cannot be solved with single-target therapies, so multiple-target approaches are needed. Core targets of AD to date are soluble oligomeric Aβ species and neuroinflammation, in an intimate detrimental dialogue. Aβ oligomers, the most neurotoxic species, appear to induce synaptic and cognitive dysfunction through the activation of glial cells. Anti-inflammatory drugs can prevent the action of Aβ oligomers. Neuroinflammation is a chronic event whose perpetuation leads to the continuous release of pro-inflammatory cytokines, promoting neuronal cell death and gross brain atrophy. Among the possible multi-target therapeutic alternatives, this review highlights the antibiotic tetracyclines, which besides antimicrobial activity also have pleiotropic action against amyloidosis, neuroinflammation, and oxidative stress. A particular focus will be on doxycycline (Doxy), a second-generation tetracycline that crosses the blood-brain barrier more easily and has a safer clinical profile. Doxy emerged as a promising preventive strategy in prion diseases and gave compelling pre-clinical results in mouse models of AD against Aβ oligomers and neuroinflammation. This strongly supports its therapeutic potential and calls for deciphering its exact mechanisms of action so as to maximize its effects in the clinic.”
Coupling what is said in the above three articles. It appears that Doxycycline can contribute to neurogenesis, reduction of neuroinflammation, and even possibly to reduction of misfolded proteins.
So is doxycycline an important longevity drug, or simply another entry in a long list of substances that appear to have some longevity benefit? I think it is too soon to say yet. I will be looking for unusual results of my current doxycline treatment in coming weeks and may get back to this topic in subsequent blog entries. When I started writing this blog, I had no idea that doxycycline would come up!
So where does this leave us?
There is a great deal more that can be said about the subjects of this and the previous blog, and I will likely return to some of these topics soon.
As a reader of advancing age you might well ask “What are the practical things this and the previous blog entry tell me I might do to prevent or resolve chronic neuroinflammation? I plan to address important aspects of that question in a soon-to-come blog entry on what I actually have been and am doing for myself. Specifically I plan to re-list the dietary supplements I am taking and, in each case, state what benefits I am expecting from that supplement and how it relates to my current health. And yes, you will now find certain mushroom extracts on that list.
MEDICAL DISCLAIMER
FROM TIME TO TIME, THIS BLOG DISCUSSES DISEASE PROCESSES. THE INTENTION OF THOSE DISCUSSIONS IS TO CONVEY CURRENT RESEARCH FINDINGS AND OPINIONS, NOT TO GIVE MEDICAL ADVICE. THE INFORMATION IN POSTS IN THIS BLOG IS NOT A SUBSTITUTE FOR A LICENSED PHYSICIAN’S MEDICAL ADVICE. IF ANY ADVICE, OPINIONS, OR INSTRUCTIONS HEREIN CONFLICT WITH THAT OF A TREATING LICENSED PHYSICIAN, DEFER TO THE OPINION OF THE PHYSICIAN. THIS INFORMATION IS INTENDED FOR PEOPLE IN GOOD HEALTH. IT IS THE READER’S RESPONSIBILITY TO KNOW HIS OR HER MEDICAL HISTORY AND ENSURE THAT ACTIONS OR SUPPLEMENTS HE OR SHE TAKES DO NOT CREATE AN ADVERSE REACTION.
View the full article at Anti-Aging Firewalls









































