.
O P E N A C C E S S S O U R C E : AgING
Abstract
Stable transfection manipulation with antibiotic selection and passaging induces progressive cellular senescence phenotypes. However, the underlying mechanisms remain poorly understood. This study demonstrated that stable transfection of the empty vector induced PANC-1 cells into cellular senescence. Metabolomics revealed several acylcarnitines and their upstream regulatory gene, carnitine palmitoyltransferase 1C (CPT1C) involved in fatty acid β-oxidation in mitochondria, were strikingly decreased in senescent PANC-1 cells. Low CPT1C expression triggered mitochondrial dysfunction, inhibited telomere elongation, impaired cell survival under metabolic stress, and hindered the malignance and tumorigenesis of senescent cells. On the contrary, mitochondrial activity was restored by CPT1C gain-of-function in senescent vector PANC-1 cells. PPARα and TP53/CDKN1A, crucial signaling components in cellular senescence, were downregulated in senescent PANC-1 cells. This study identifies CPT1C as a key regulator of stable transfection-induced progressive PANC-1 cell senescence that inhibits mitochondrial function-associated metabolic reprogramming. These findings confirm the need to identify cell culture alterations after stable transfection, particularly when cells are used for metabolomics and mitochondria-associated studies, and suggest inhibition of CPT1C could be a promising target to intervene pancreatic tumorigenesis.
Introduction
A commonly used stable transfection manipulation for an exogenous gene with a selectable marker that remains in the genome of eukaryotic cells and their daughter cells is highly desirable [1]. Cells then remain alive and can be further cultivated under selective stress with passaging. However, stable transfection manipulation induces progressive cellular senescence phenotypes, leading to a common problem in cell culture. The underlying mechanisms of stable transfection-induced cellular senescence remain poorly understood. As reported in our previous study [2], extended passaging of PANC-1 cells were triggered into a replicative senescence process with low CPT1C levels. Low CPT1C expression further caused mitochondria dysfunction-associated metabolic reprogramming and impaired malignancy in senescent replicative PANC-1 cells. More importantly, knockdown of CPT1C in cancer cells induced mitochondrial dysfunction, senescence-like growth suppression and cellular senescence and further suppressed malignancy and tumorigenesis in vivo and xenograft tumor growth in situ. On the contrary, the gain-of-function of CPT1C reversed PANC-1 cell senescence and enhanced mitochondrial function. CPT1C was hence confirmed as a novel biomarker for mitochondrial dysfunction-associated cellular senescence [2]. Recently, microRNA-1291 and its mimic empty vector pCMV stably transfected PANC-1 cell lines were established to reveal the role of microRNA-1291 in pancreatic carcinoma cell metabolism and suppressed tumorigenesis [3]. It was found that stable transfection manipulation induces progressive PANC-1 cell senescence, but whether the underlying mechanisms of this senescence are mitochondrial dysfunction-associated or CPT1C-depent remain unclear. Therefore, the current study aimed to study the metabolomics change in stable transfection-induced progressive PANC-1 cell senescence and to reveal the underlying molecular signals involved in this progress. The metabolomics results demonstrate several acylcarnitines and their upstream regulatory gene, CPT1C, were strikingly decreased in senescent PANC-1 cells. CPT1C was further identified as a crucial regulator in stable transfection-induced PANC-1 cell senescence by inhibiting mitochondrial function-associated metabolic reprogramming. These findings suggest the need to identify cell culture alterations after stable transfection, particularly when cells are used for metabolomics and mitochondria-associated studies, and suggest inhibition of CPT1C could be a promising target to intervene pancreatic tumorigenesis.
Results
Stable transfection-induced PANC-1 cell senescence
In our previous study, microRNA-1291 and its mimic empty vector pCMV stably transfected PANC-1 cell lines were established to reveal the role of microRNA-1291 in pancreatic carcinoma cell metabolism and suppressed tumorigenesis [3]. Here, it was confirmed that stable transfection of the empty vector pCMV in human pancreatic epithelioid carcinoma PANC-1 cells led to severe growth arrest and cellular senescence.
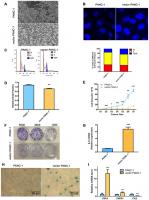
Vector PANC-1 cells (PANC-1 cells stably transfected with the empty vector pCMV were characterized by an enlarged and flattened appearance arranged like flagstones with increased granularities in the cytoplasm (Figure 1A) compared with mock PANC-1 cells (untreated PANC-1 cells). Consistent with this result, degenerative changes of enlarged nuclei were also observed in vector PANC-1 cells (Figure 1B). To examine cell growth suppression of the senescence-associated phenotypes, flow cytometry was performed to determine whether stable transfection of the vector caused an increase in the population of vector PANC-1 cells in G2/M phase (Figure 1C). Vector PANC-1 cells exhibited lower proliferation than mock PANC-1 cells, as revealed by BrdU incorporation measured during DNA synthesis (Figure 1D) and cell growth curve tracing (Figure 1E). Furthermore, a weaker ability of vector PANC-1 cells to form cell colonies was observed (Figure 1F). IL-8, a key SA secretory phenotype (SASP) factor involved in the senescence process [4–7], was increased in vector PANC-1 cells compared to mock PANC-1 cells (Figure 1G). IL-8 was negatively correlated (but without statistical significance) with CPT1C mRNA expression in pancreatic cancer patients (Supplementary Figure 2A), further supporting enhanced SASP in low-CPT1C-induced senescent vector PANC-1 cells. More importantly, β-galactosidase (SA-β-gal) staining showed that mock PANC-1 cells were nearly negative for β-gal, while vector PANC-1 cells were positive for senescent signals (Figure 1H). The mRNA levels of TNF-α and its receptor TNFR1, were significantly increased in vector PANC-1, indicating the activation of extrinsic apoptosis pathways in the senescent cells. However, FAS mRNA expression was reduced in the senescent cells, which might result from the negative feedback regulation of activation of TNF-α-TNFR1 pathway (Figure 1I).
Figure 1. Stable transfection-induced PANC-1 cell senescence. (A) Morphology graph of vector PANC-1 cells. (B) Confocal fluorescent graph of the nuclei (blue fluorescence) morphology of vector PANC-1 cells. © An increased percentage of vector PANC-1 cells was arrested in G2/M phase. Graphic (top) and percentage (bottom) representations of cell cycle distributions are shown. This experiment was repeated independently three times. (D) Decreased BrdU incorporation during DNA synthesis in vector PANC-1 cells. Data are presented as the mean ± S.E.M, n = 4 (**p < 0.01). (E) Cell growth curve shows decreased proliferation of vector PANC-1 cells. Data are presented as the mean ± S.E.M, n = 3 (*p < 0.05, **p < 0.01, ***p < 0.001). (F) Decreased ability of vector PANC-1 cells to form colonies when seeded at the indicated dilutions. (G) Quantitative RT-PCR analysis of the upregulated key SASP factor, IL-8 mRNA, in vector PANC-1 cells. Data are presented as the mean ± S.E.M, n = 3 (***p < 0.001). (H) SA-β-gal staining and positive senescence signal of vector PANC-1 cells. This experiment was repeated independently three times. (I) Activation of extrinsic apoptosis pathways was analyzed. See also Supplementary Figures 1 and 2.
Taken together, these data indicate that stable transfection of the empty vector triggered PANC-1 cells into a strong senescence-like growth suppression and severe cellular senescence.
Metabolomics reveals a lower level of acylcarnitines in senescent vector PANC-1 cells, which is linked to reduced CPT1C expression
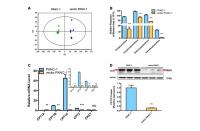
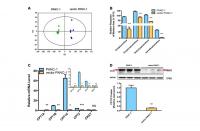
Metabolomics analysis was performed to further identify potential regulators or biomarkers underlying cellular senescence induced by stable transfection of the empty vector pCMV. To identify the general trends in an unbiased way, unsupervised principal component analysis (PCA) was performed to reveal differences between the mock and vector PANC-1 cells. PCA scatter diagrams obtained from HILIC-ESI+-MS (Figure 2A) and HILIC-ESI—MS (Supplementary Figure 3A) showed a clear separation between the mock and vector PANC-1 cells, suggesting a distinct discrimination in the metabolome profiles between these two groups. S-plot of OPLS/DA models resulting from HILIC-ESI+- MS indicated four significantly changed ions (Supplementary Figure 3B). The ions were further specifically identified as acetylcarnitine (Supplementary Figure 3C), propionylcarnitine (Supplementary Figure 3D), isobutyrylcarnitine (Supplementary Figure 3E) and isovalerylcarnitine (Supplementary Figure 3F). Interestingly, the relative response of all of the marker ions was significantly reduced in senescent vector PANC-1 cells (Figure 2B).
Figure 2. Metabolomics reveals a lower level of acylcarnitines in senescent vector PANC-1 cells, which is linked to reduced CPT1C expression. (A) PCA score plots of HILIC-ESI+-MS metabolomics profiles obtained from HILIC-ESI+-MS, n = 6/group. (B) Analysis of the relative response of acylcarnitine ions in senescent vector PANC-1 cells. Data are presented as the mean ± S.E.M, n = 6 (***p < 0.001). © Quantitative RT-PCR analysis of genes related to acylcarnitines. Data are presented as the mean ± S.E.M, n = 3 (ns indicates no significance, *p < 0.05, **p < 0.01, ***p < 0.001). The specific human primers to amplify corresponding mRNA were obtained from website of http://pga.mgh.harvard.edu/primerbank/ and PrimerDepot, and commercially available (Invitrogen) and shown in Supplementary Table 1. (D) Images and densitometric analysis of CPT1C protein bands of senescent vector PANC-1 cells. Data are presented as the mean ± S.E.M, n = 3 (**p < 0.01). See also Supplementary Figure 3.
To identify the potential drivers behind the dramatic decrease in acylcarnitine levels in senescent vector PANC-1 cells, the mRNA expression of genes involved in acylcarnitine transport was further determined. Specifically, CPT1B and CPT2 mRNA levels were significantly decreased in vector PANC-1 cells, while CPT1A mRNA levels showed a slight increase and carnitine O-acetyltransferase (CRAT) mRNA expression remained unchanged (Figure 2C). However, CPT1C mRNA levels were the most strikingly decreased in vector PANC-1 cells compared to mock PANC-1 cells (Figure 2C). Furthermore, CPT1C protein levels were significantly reduced in senescent vector PANC-1 cells (Figure 2D). Together, these data imply that the decrease in CPT1C is the most important contributor to the transport of decreased acylcarnitines and may represent a driver of vector PANC-1 senescence.
Dysfunctional mitochondria and inhibited telomere elongation in low-CPT1C-expressing senescent vector PANC-1 cells
CPT1C catalyzes the transportation of fatty acids from the cytoplasm to the mitochondrial matrix for β-oxidation in the mitochondrial outer member. Mitochondrial function was further examined in senescent vector PANC-1 cells. Notably, vector PANC-1 cells produced significantly less ATP under conditions of culture medium deprivation (Figure 3A). Moreover, the reduced intensity of absorbed rh123 dye indicated the loss of mitochondrial transmembrane permeability upon low CPT1C expression in vector PANC-1 cells (Figure 3B).
Figure 3. Dysfunctional mitochondria in low-CPT1C-expressing senescent vector PANC-1 cells. (A) ATP production in senescent vector PANC-1 cells, the magnitude of this difference increased as the time in PBS was extended to 24 h. Data are presented as the mean ± S.E.M, n = 4 (**p < 0.01, ***p < 0.001). (B) Loss of mitochondrial transmembrane potential measured by the rh123 dequenching method in senescent vector PANC-1 cells. Data are presented as the mean ± S.E.M, n = 4 (**p < 0.01). © Mitochondrial integrity in the forms of OCRs (pMol O2.min-1) in senescent vector PANC-1 cells. Data are presented as the mean ± S.E.M, n = 3. (D) Maximal respiration capacity in the form of OCRs in senescent vector PANC-1 cells. Data are presented as the mean ± S.E.M, n = 3 (**p < 0.01). (E) Spare respiratory capacity in the form of OCRs in senescent vector PANC-1 cells. Data are presented as the mean ± S.E.M, n = 3 (***p < 0.001). (F) Mitochondriogenesis analysis in senescent vector PANC-1 cells. Data are presented as the mean ± S.E.M, n = 3 (*p < 0.05, ***p < 0.001). (G) The mitochondrial network structure integrity analysis on the senescent cells. Data are presented as the mean ± S.E.M, n = 3 (***p < 0.001). (H) Mitochondrial autophagy analysis on the senescent cells. Data are presented as the mean ± S.E.M, n = 3 (**p < 0.01, ***p < 0.001). (I) Mitochondriogenesis analysis on senescent vector PANC-1 cells gaining of CPT1C function. Data are represented as mean ± S.E.M, n = 4 (*p< 0.05). See also Supplementary Figure 4.
Oxygen consumption rates (OCRs) from vector PANC-1 cells exhibited lower maximal respiration after the injection of FCCP than mock PANC-1 cells (Figure 3C, 3D). More importantly, the spare respiratory capacity of vector PANC-1 cells was significantly decreased (Figure 3C, 3E), which was indicative of a reduced ability to respond to an increased energy demand. However, vector PANC-1 cells also exhibited higher basal respiratory rates (Figure 3E) and glycolytic function in the forms of extracellular acidification rates (ECARs) (Supplementary Figure 4A), implying that the cells may adjust to mitochondrial respiration to maintain energy supply homeostasis under a long-term stable transfection-induced progressive senescence program [8]. Together, partially impaired mitochondrial respiration integrity upon low CPT1C expression was observed in senescent vector PANC-1 cells.
PGC-1a mRNA levels were reduced in vector PANC-1 cells, and its downstream genes, NRF-1 and TFAM mRNAs, were also decreased. CYTB, one of the representative mtDNA-encoded subunits [9], was subsequently lowered (Figure 3F).
Genes encoding mitochondrial fission and fusion proteins, including MFN1, MFN2 and OPA1, were significantly reduced in senescent vector PANC-1 cells (Figure 3G), suggesting the mitochondrial network structure integrity was impaired in the senescent cells, which might further explain the impaired mitochondrial respiration and mitochondriogenesis pathways. PRNK, encoding PARKIN protein, was also reduced, while PINK1 mRNA expression increased in the senescent cells (Figure 3H). Combined with Figure 3G, these data suggested that mitochondrial autophagy (mitophagy) process might be hampered and a negative feedback might exist.
On the contrary, enhanced cellular bioenergetics pathways (Figure 3I) were observed in the senescent cells overexpressing CPT1C, which suggested mitochondrial function was restored by CPT1C gain-of-function.
Additionally, the shortening or structural changes of telomeres at the ends of the chromosomes leads to a DNA damage response and ultimately triggers replicative senescence [10]. Shown as Figure 4A, stable transfection of the vector pCMV remarkably inhibited telomerase activity. As a consequence, the length of the telomere in senescent vector PANC-1 cells shortened by approximately 0.9 kb (Figure 4B).
.../...
.