Induced pluripotent stem cells (iPS) have been one of the most exciting developments in the rejuvenation biotechnology field. In addition to being a fascinating biological phenomenon, the ability to reprogram adult somatic cells to stem cells that appear to have the full range of properties as embryonic stem cells (ESC) offers great biommedical promise: a way to generate autologous cells for cell therapy and tissue engineering with the same pluripotent potential as ESC, but without their potential for immunological rejection; without some of the practical hurdles (such as oocyte supply and mitochondrial incompatibility) that might accompany use of somatic cell nuclear transfer (SCNT -- "theraputic cloning") in humans; and bypassing the misguided "ethical" confusion and political hurdles facing the development and use of either of the two earlier methods.
Progress in the field has been rapid, as we have highlighted in several updates along the way, and the popular and nonspecialist scientific media have been unusually consistent and in-depth in their coverage.
However, in some ways there has been too much emphasis placed on these cells. The promise of the cells has some times been inflated into a definitive statement that iPS cells are of equal potential to ESC and SCNT, and this popular notion has been exploited by misguided political opponents of ESC and SCNT to abandon research into these cells, in favor of the new reprogramming technology. Even some stem cell researchers and sincere advocates of advancing stem cell research have sometimes become so eager to embrace the potential of iPS cells as to begin to deprive needed support and research from critical, ongoing work in characterizing, deriving, and developing ESC and SCNT to full therapeutic potential.
In fact, however, there remain not only the well-publicized technical hurdles in derivation and future clinical translation of iPS cells, but significant sources of uncertainty as to whether iPS cells do, in fact, have the full biological and therapeutic potential of ESC and SCNT. Amongst the most important such reason for caution has been the uncertainty of the core claim of the full pluripotency for these cells. Researchers have used a variety of different assays to evaluate the pluripotency of cell lines: the formation of teratoma that include cells from all three germ layers; the ability to create chimeras when injected into blastocysts derived from normal fertilized oocytes; and transmission of iPS-derived cells from such animals through the germline into offspring. However, the most stringent test of iPS pluripotency was initially elusive, and remains difficult to demonstrate: the ability to generate mice derived entirely from iPS cells, by injecting them into 4N blastocysts (so-called "tetraploid (4N) embryo complementation"), so that only iPS-derived cells contribute to the soma of the ensuing embryo.
This ultimate test is routinely met by ESC- and SCNT-derived 4N complementation embryos, which despite early doubts appear to generate embryos that have normal life expectancies. By contrast, in early studies, iPS-derived 4N complementation embryos could not achieve this goalpost unaided; later studies, which screened iPS cells to isolate the minority that exhibited reactivation of the pluripotency genes Oct4 and Nanog, still resulted in iPS-derived embryos that could survive through just 14.5 d of development.(1) Even in studies using the more readily-reprogrammable fetal-tissue-derived iPS, using the most recent protocols, replacing fetal bovine serum with knockout serum replacement during stem cell derivation followed by painstaking gene expression, karyotypic, and phenotypic screening, reports indicate that less than a third of iPS-derived 4N complementation embryos survive to adulthood(2) -- a very low fraction of the originally-induced cells, when one considers the very low efficiency of the reprogramming protocols in use to date. Underscoring this substantial difference between ESC or SCNT (on the one hand) and iPS cells, recent comparative studies of the gene expression profiles of iPS with ESCs have uncovered substantial and wide-ranging differences, not only between iPS and other pluripotent cells, but even amongst cell lines derived using the same core technology at different labs, with no clear evidence of an unifying, upstream regulator of the wide range of variances between the cell types.(3-6)
Matthias Stadtfeld and colleagues in Konrad Hochedlinger's lab at the Harvard Stem Cell Institute have now given us the first real insight into the factors separating iPS cells from their classical and unambiguously pluripotent cousins.(7) Beginning from the retrospectively-obvious hypothesis that the variation in gene expression between ESC and iPS in previous reports might be due to the differing background strain of the comparator cells, they directly compared ESC to iPS cells derived from the tissues of mice that had been generated from those same ESC, modified with a polycistronic cassette encoding the Yamanaka iPS genes under doxycycline-inducible expression. Remarkably, there were significant and consistent differences in expression in just 2 genes (Gtl2 and Rian, non-coding genes previously reported to remain silenced in most iPS lines) and 21 miRNAs -- an apparent culling of more than 2 orders of magnitude of artifactual incongruities between ESC and iPS observed in previous studies using cells of heterogeneous genetic background. Moreover, both the genes and the miRNAs localized to the same genomic locus: the Dlk1-Dio3 imprinted gene cluster on the murine chromosome 12qF1.(7)
To determine the generality of Gtl2 silencing in iPSCs, we measured its expression in 61 additional iPSC lines derived from haematopoietic stem cells (11 lines), granulocyte-macrophage progenitors (11 lines), granulocytes (9 lines), peritoneal fibroblasts (6 lines), tail-tip fibroblasts (6 lines) and keratinocytes (18 lines). Only four of these lines (5.8%), originating from either peritoneal or tail-tip fibroblasts, showed Gtl2 expression levels similar to those of ES cells (termed ‘Gtl2on clones’). The finding that the vast majority of iPSC clones showed transcriptional suppression of Gtl2 (termed ‘Gtl2off clones’) demonstrates that silencing of this locus occurs in iPSCs derived from different cell types at various stages of differentiation. Analysis of published microarray data sets comparing ES cells and iPSCs generated from mouse fibroblasts, neural and bone marrow cells also showed repression of maternally expressed 12qF1 transcripts, supporting the notion that silencing of this cluster is a common outcome upon factor-mediated reprogramming. (7)
Gtl2 expression or repression remained stable through subcloning of the various iPS lines, even when doxycycline was reintroduced during the subcloning process itself, and also persisted when differentiation was induced with retinoic acid. Moreover, a review of previously-characterized SCNT lines capable of 4N complementation showed that they, too, exhibited Gtl2 expression.
To evaluate the effect of the aberrant silencing of these genes on the pluripotency of iPS lines, Stadfeldt et al proceeded to generate chimeras and 4N-complementation embryos from Gtl2on and Gtl2off iPS cells. Animals generated from Gtl2off cells exhibited low (10–50%) levels of coat-color chimerism, while Gtl2on clones supported chimerism rates similar to those observed with ESC (30-100%). And while both kinds of lines could bring 4N-complementation blastocysts to embryonic day 11.5 with superficially normal phenotype, none of ten Gtl2off lines were able to generate viable pups; not even nuclear transfer could reset the silencing of Gtl2 and Rian in Gtl2off iPSC, and cells thus derived remained obstinately 4N-complementation incompetent. By contrast, all of four 4N-complementation embryos derived from Gtl2on iPS clones supported all-iPS mice, with efficiencies typical of ES (7–19% vs. 13–20% efficiency). "To our knowledge, this is the first demonstration of animals produced entirely from adult-derived iPSCs"(7) (Figure 1).
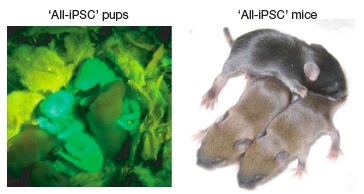
Figure 1: First Mice Produced Entirely From Adult-Derived iPS. Reproduced from (7).
Finally, the investigators used the histone deacetylase inhibitor valproic acid to deacetylate the Gtl2 locus of Gtl2off iPS subclones. In 2 of 21 Gtl2off subclones, Gtl2 expression increased, and in one of these the level approximated that of ESC. At embrynic day 11.5, 4N-complementation embryos derived from such cells were superficially phenotypically normal at comparable rates to natively Gtl2on clones; and unlike the unmodified clones, some of the embryos from valproic-acid-treated Gtl2off clones went on to develop into full-term all-iPSC pups. These embryos expressed not only Gtl2 and Rian, but normal expression of tissue-specific marker genes that were repressed in Gtl2off embryos. Valproic acid treatment did not however lead to normal pups: they were "severely overgrown" (Fig. 2 below) and not ultimately viable. The authors hypothesize that valproic acid treatment may have led to overexpression of Dlk1, which generates a similar, fatal phenotype when it occurs spontaneously; alternatively, the drug may have aberrantly deacetylated non-target genes.(7)
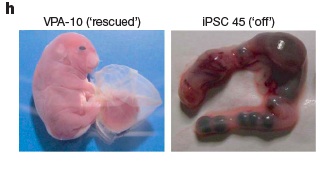
Figure 2: Valproic Acid Advances Development of Gtl2off iPS-derived Embryos. Reproduced from (7).
The challenge of deriving fully pluripotent cells using the new reprogramming technology has been substantial, and will continue for some time -- perhaps indefinitely -- to be substantial. Well-justified excitement over these cells have sometimes led the popular media, some stem cell advocates, and misguided opponents of ESC and SCNT research and therapeutic development to gloss over the magnitude of these difficulties. This (7) is an important study, and will doubtless make a key contribution to our ultimate ability to harness iPS cells for rejuvenating aging tissues. But the limitations that remain with reprogrammed cells powers relative to those of their cousins, and the need for comparison studies to identify the basis for these differences and to work toward overcoming them, must emphasize the need to continue vigorously advancing research using all varieties of pluripotent (or potentially pluripotent) stem cells. The political and scientific challenges continue, and we have a scientific and moral duty to press on, for the lives and health that stand in the balance.
References
1: Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007 Jul 19;448(7151):318-24. Epub 2007 Jun 6. PubMed PMID: 17554336.
2: Zhao XY, Li W, Lv Z, Liu L, Tong M, Hai T, Hao J, Guo CL, Ma QW, Wang L, Zeng F, Zhou Q. iPS cells produce viable mice through tetraploid complementation. Nature. 2009 Sep 3;461(7260):86-90. PubMed PMID: 19672241.
3: Chin MH, Pellegrini M, Plath K, Lowry WE. Molecular analyses of human induced pluripotent stem cells and embryonic stem cells. Cell Stem Cell. 2010 Aug 6;7(2):263-9. PubMed PMID: 20682452.
4: Newman AM, Cooper JB. Lab-specific gene expression signatures in pluripotent stem cells. Cell Stem Cell. 2010 Aug 6;7(2):258-62. PubMed PMID: 20682451.
5: Guenther MG, Frampton GM, Soldner F, Hockemeyer D, Mitalipova M, Jaenisch R, Young RA. Chromatin structure and gene expression programs of human embryonic and induced pluripotent stem cells. Cell Stem Cell. 2010 Aug 6;7(2):249-57. PubMed PMID: 20682450.
6: Stadtfeld M, Maherali N, Borkent M, Hochedlinger K. A reprogrammable mouse strain from gene-targeted embryonic stem cells. Nat Methods. 2010 Jan;7(1):53-5. Epub 2009 Dec 13. PubMed PMID: 20010832.
7: Stadtfeld M, Apostolou E, Akutsu H, Fukuda A, Follett P, Natesan S, Kono T, Shioda T, Hochedlinger K. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010 May 13;465(7295):175-81. Epub 2010 Apr 25. PubMed PMID: 20418860.
View the full article












































