Sounds like Google's company is on top of this one.
If I recall correctly, the backdrop of that discussion I mentioned was resveratrol and the SIRT1 resveratrol craze at the time. That discussion around that time by niner, geddarkstorm and others prompted evaluation of AMPK activators as a way to increase NAMPT, increase NAD+ and improve sirtuins.
Example thread
Example thread
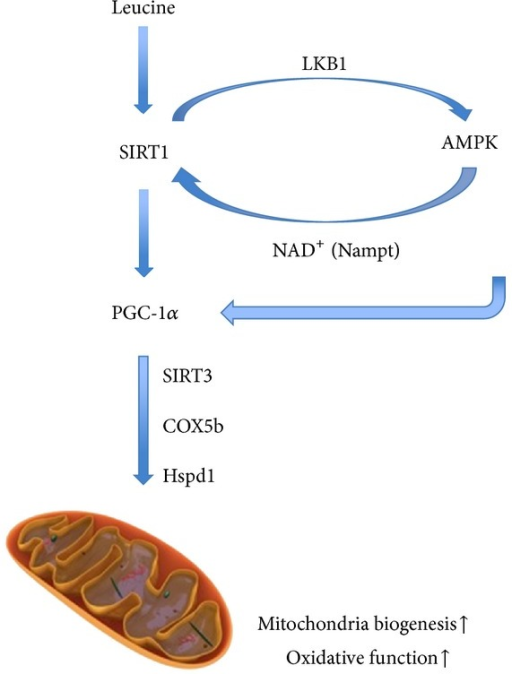
The best solution is to upregulate AMPK through what we know are obvious approaches: periodic fasting and exercise (in a fasted state). But the first drug/supplement that came to my mind then... and still does today is AICAR.
AICAR --> AMPK --> Nampt/NAD --> Sirt1/3 activation
I'm still kind of amazed there was never a group buy for AICAR and a lack of interest in it, given all the purchases that have gone on in this forum over the years. I may be making this too simplistic, but to me, AICAR is basically an injectable form (peptide) of aerobic exercise. Maybe I'm missing something very basic about it or alternatives like bitter melon extract or metformin were just easily obtainable and not some injectable drug.
Edited by tunt01, 03 September 2018 - 03:58 PM.































 This topic is locked
This topic is locked

















