Astragalus, Astragaloside IV
#1561
Posted 12 December 2011 - 06:16 PM
Soo who is the most legit of the two? Seems otherwise obvious Crackagin since one get 32% more...
Any idea why the same price but different purities, any of the site more reliable? Only Terraternal show the labs report results.
#1562
Posted 13 December 2011 - 03:09 AM
Greenpower, you have consistently provided the most valuable, thorough, objective and exhaustive information on this forum.
Thank you. Did you take any breaks from the extract, cycle it in any way or stayed on it consistently? Thanks again for all your input.
Thanks, I try to apply some basic "test principles" and also be as objective as possible. During a period which has been as long as the last one, it's been hard to keep exact cycling-periods, but I try to do "three months on - three weeks off".
Btw, I got some anomalies in my Melatonin and Adrenocortex stress profiles which i will report as soon as I have a spare moment.
I also want to 2nd that: Greenpower, you've done more with your rigorous approach and detailed progression than we can thank you for. I might be living (possibly) 10 years or more longer, healthier, with the benefit of your sharing of results.
I owe you a pint! or maybe even a mixed fruit smoothie!! (could be healthier!!)
#1563
Posted 18 December 2011 - 09:11 PM
Cortisol
Last time the results where under the reference range during noon. This time they were normal during the whole day.
Cortisol and DHEA
Last time the cortisol results were within the reference ranges during the whole day. This time cortisol levels where also within the reference ranges during the whole day, but the concentration during the morning had more than doubled since last time and was way up in the yellow territory.
The DHEA and DHEA/Cortisol ratio were within the reference ranges but went from bordering on being to high, to reaching the lower borders.
I think this might be the results of "too much work and too little sleep".
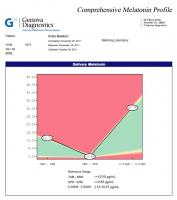
Melatonin
Last time the values were within the reference range, although bordering on being too high in the morning. This time it was to high both in the morning and during the night. The lab had the following to say about this:
Both 7-9 AM and 2:30-3:30 AM melatonin levels are elevated.
High morning melatonin levels are often present in individuals with Seasonal Affective Disorder. This may be due to prolonged nocturnal production of melatonin, and/or late onset of its production. High melatonin levels may bring about inhibition of ovulation in women as well as decreased body temperature. High melatonin has been noted in the manic phase of bipolar mood disorder. Many antidepressant drugs may stimulate melatonin production, including fluvoxamine (Luvox), desipramine, and most MAO inhibitors. Prozac may lower melatonin levels.
This profile reveals a disturbance in the circadian rhythm of melatonin. This may influence other hormones such as thyroid, testosterone, and estrogen. As well as playing a crucial role in sleep-wake cycles, melatonin influences other vital functions including cardiovascular and antioxidant protection, endocrine function, immune regulation and body temperature.
It would be interesting to know which type of immune regulation this would have. I have started to have cold hands and feet some time, but I can't say I've been feeling manic. I've long thought that my regimen have some properties similar to antidepressants, this might be an indication that so is actually the case. Or not. It might just be that I sometimes have been so tired and overworked that I've just dropped dead in bed and slept very deep.
Summary
My current theory is that my Natural Killer cells are very susceptible to too little sleep and to much stress. I will continue with the same regimen as I've been doing for the last 18 months, but try to sleep more and work less. Hopefully this will show positive results for the NK's during my next test run.
sponsored ad
#1564
Posted 19 December 2011 - 03:54 PM
http://www.revgenetics.com/wp/
In the early 1990’s it was discovered that the reason human cells become “old” and are unable to continue growing like they do when they are “young” was due to shortening of the ends of the DNA regions called the telomeres. As it was first discovered in other organisms and then in humans, the telomeres shorten every time the cell undergoes cell division. When a critical telomere length is reached, a signal was created that would then stop the cell from replicating, at this point the cell was referred as senescent. However, many questions remained for example, what exactly was causing the signal that actually stopped the cells from replicating, what proteins were involved and could these signals be stopped or reversed. Since then many proteins involved with regulating telomere length have been discovered, most notably telomerase, which can directly extend the telomeres. However, a remaining question from this early research was the observation that humans have 23 chromosome pairs (i.e. DNA) and each has a different telomere length. Could it be possible that a single chromosome shortening stop the cell from dividing or would it be a combine signal from a few or all chromosomes? These questions were finally addressed by a research group from Australia and published in the journal of EMBO reports (EMBO reports, December 2011, pages 1-8). The authors discovered that five telomeres that had reached critical length were required before the signals associated with senescence were triggered. This research may seem trivial given the fact that methods of extending telomeres exist. But when one is reminded that historically research on aging was viewed as trivial because of its inevitability, and now it is an active area of research. This research may point to the importance of looking for specific telomerase activators that target the shortest of telomeres and delay the aging of our cells.
Although his post is meant for folks who have little to no knowledge of the subject, If you have any specific questions for Dr. Valenzuela, that he can answer on his blog or in the newsletter, I will forward them on.
Cheers
A
#1565
Posted 20 December 2011 - 01:38 AM
#1566
Posted 05 January 2012 - 08:18 PM
However, this may prove to be an issue with bioavailability if we have cycloastragenol formed only in the the large intestine.
If the later is the case, I could see the importance of using the cycloastragenol form (with plant sugars removed) for greater bioavailability at the outset.
So the reason I think T.A. Sciences is using TA-65 in pure form, probably to insure careful control over dosage and purity of product, eliminating unknowns in "The Patton Protocol" study. Why would you pay all your hard earned money out, just buy some inexpensive astragaloside sugars in a few capsules and swallow down along with a couple of beano’s. The beanos could cleave off the glycone groups in situ and viola your cycloastragenol.
“Long Live You and Your Money”
Chris h
see http://en.wikipedia....side_hydrolases
Also
http://en.wikipedia....ary_supplement)
#1567
Posted 19 January 2012 - 06:55 PM
A Quick Entry From Dr. Valenzuela:
http://www.revgenetics.com/wp/In the early 1990’s it was discovered that the reason human cells become “old” and are unable to continue growing like they do when they are “young” was due to shortening of the ends of the DNA regions called the telomeres. As it was first discovered in other organisms and then in humans, the telomeres shorten every time the cell undergoes cell division. When a critical telomere length is reached, a signal was created that would then stop the cell from replicating, at this point the cell was referred as senescent. However, many questions remained for example, what exactly was causing the signal that actually stopped the cells from replicating, what proteins were involved and could these signals be stopped or reversed. Since then many proteins involved with regulating telomere length have been discovered, most notably telomerase, which can directly extend the telomeres. However, a remaining question from this early research was the observation that humans have 23 chromosome pairs (i.e. DNA) and each has a different telomere length. Could it be possible that a single chromosome shortening stop the cell from dividing or would it be a combine signal from a few or all chromosomes? These questions were finally addressed by a research group from Australia and published in the journal of EMBO reports (EMBO reports, December 2011, pages 1-8). The authors discovered that five telomeres that had reached critical length were required before the signals associated with senescence were triggered. This research may seem trivial given the fact that methods of extending telomeres exist. But when one is reminded that historically research on aging was viewed as trivial because of its inevitability, and now it is an active area of research. This research may point to the importance of looking for specific telomerase activators that target the shortest of telomeres and delay the aging of our cells.
Although his post is meant for folks who have little to no knowledge of the subject, If you have any specific questions for Dr. Valenzuela, that he can answer on his blog or in the newsletter, I will forward them on.
Cheers
A
I have a question. Is there any reason to believe that extending the telomeres of a cell will have a negative overall health effect? For instance, due to the fact that the internal organelles of the cell have deteriorated from an optimal condition that has nothing to do with telomeres (e.g. mitochondrial disfunction)?
#1568
Posted 19 January 2012 - 10:20 PM
http://arthritis-res.../1/R12/abstract
see also:
http://www.ncbi.nlm....pubmed/21291897
http://www.ncbi.nlm....pubmed/20971668
for more on the same condition
#1569
Posted 20 January 2012 - 12:28 AM
I have a question. Is there any reason to believe that extending the telomeres of a cell will have a negative overall health effect? For instance, due to the fact that the internal organelles of the cell have deteriorated from an optimal condition that has nothing to do with telomeres (e.g. mitochondrial disfunction)?
Most cancerous cells have telomerase that is constitutively active; that is, it is mutated such that it is stuck in the "on" position. If a cell were cancerous but didn't have active telomerase, then you wouln't want to have it turned on. If a cell is already badly damaged, it would ideally commit suicide via apoptosis. Less ideally, it might go senescent. Lengthening telomeres probably wouldn't have much effect on either condition. If a cell had gone senescent because of critically short telomeres, and it was also badly damaged, but not bad enough to cause senescence, then if you lengthen the telomeres, you'd get a damaged but non-senescent cell. Whether that's bad or not would depend on the damage. Senescent cells are bad because they pump out inflammatory compounds. Random damage to a cell can manifest in a lot of ways, the most dangerous of which is cancer.
#1570
Posted 20 January 2012 - 01:20 AM
I have a question. Is there any reason to believe that extending the telomeres of a cell will have a negative overall health effect? For instance, due to the fact that the internal organelles of the cell have deteriorated from an optimal condition that has nothing to do with telomeres (e.g. mitochondrial disfunction)?
Shorten telomeres are linked to bad health; this has been demonstrated by many studies. Whereas, long telomeres have been closely linked with better health, function and youth. Typically, a cell that has shorten telomeres indicates that the cell has undergone multiple rounds of cell division and is getting old, by then other organelles such as the mitochondria will also begin to malfunction. These events in addition to other cell changes will occur together as the normal process of aging. However, they sometimes occur at slightly different stages. Think of an old car. The engine starts to leak oil and the tires get worn out. The oil leak may be more serious in the long-term. It you can prevent your engine from leaking oil you just might get a few extra miles out of the car. Keeping long telomere is key for a cell’s longevity. So keeping long telomeres is most likely to give our cells a few extra miles. In some cells (such as fibroblasts) being able to keep your telomere intact maintains the rest of the cell’s organelles working properly apparently endlessly.
Hector Valenzuela, Ph.D.
Chief Science Officer
RevGenetics
Edited by Anthony_Loera, 20 January 2012 - 01:22 AM.
#1571
Posted 20 January 2012 - 04:40 AM
This was emailed back to me about the question:
I have a question. Is there any reason to believe that extending the telomeres of a cell will have a negative overall health effect? For instance, due to the fact that the internal organelles of the cell have deteriorated from an optimal condition that has nothing to do with telomeres (e.g. mitochondrial disfunction)?
Shorten telomeres are linked to bad health; this has been demonstrated by many studies. Whereas, long telomeres have been closely linked with better health, function and youth. Typically, a cell that has shorten telomeres indicates that the cell has undergone multiple rounds of cell division and is getting old, by then other organelles such as the mitochondria will also begin to malfunction. These events in addition to other cell changes will occur together as the normal process of aging. However, they sometimes occur at slightly different stages. Think of an old car. The engine starts to leak oil and the tires get worn out. The oil leak may be more serious in the long-term. It you can prevent your engine from leaking oil you just might get a few extra miles out of the car. Keeping long telomere is key for a cell’s longevity. So keeping long telomeres is most likely to give our cells a few extra miles. In some cells (such as fibroblasts) being able to keep your telomere intact maintains the rest of the cell’s organelles working properly apparently endlessly.
Hector Valenzuela, Ph.D.
Chief Science Officer
RevGenetics
Thanks Anthony and Hector.
#1572
Posted 20 January 2012 - 04:46 AM
I have a question. Is there any reason to believe that extending the telomeres of a cell will have a negative overall health effect? For instance, due to the fact that the internal organelles of the cell have deteriorated from an optimal condition that has nothing to do with telomeres (e.g. mitochondrial disfunction)?
Most cancerous cells have telomerase that is constitutively active; that is, it is mutated such that it is stuck in the "on" position. If a cell were cancerous but didn't have active telomerase, then you wouln't want to have it turned on. If a cell is already badly damaged, it would ideally commit suicide via apoptosis. Less ideally, it might go senescent. Lengthening telomeres probably wouldn't have much effect on either condition. If a cell had gone senescent because of critically short telomeres, and it was also badly damaged, but not bad enough to cause senescence, then if you lengthen the telomeres, you'd get a damaged but non-senescent cell. Whether that's bad or not would depend on the damage. Senescent cells are bad because they pump out inflammatory compounds. Random damage to a cell can manifest in a lot of ways, the most dangerous of which is cancer.
Yes, this is my concern. You give a damaged cell more time to divide and that might mean more problems. I guess the hope would be that lengthening all the telomeres would still keep at least a level playing field between the good and bad cells.
#1573
Posted 25 January 2012 - 06:39 PM
It is possible that large doses of resveratrol or curcumin can inhibit telomerase, however the amount necessary is in question. Again, I will personally continue to side on behalf of caution and take inhibitors separately, but only because I do not know the actual dose in humans that can cause inhibition.
A
#1574
Posted 26 January 2012 - 02:50 PM
Howard
#1575
Posted 26 January 2012 - 02:59 PM
I believe it would affect all telomerase activators at this time, not just astragalus, unless of course it's shown otherwise. I wouldn't consider telomere support supplements to be placed in with the activators when considering cycling, unless they have shown to activate telomerase.
For example, vitamins should not be considered as something to be included in cycling, even though many consider them telomere support supplements (because of previous studies showing folks taking vitamins, have less telomere attrition than those who don't take vitamins).
I do recommend cycling your intake of telomerase activators at this point.
Suggestions for Cycling? (also considered as a scheduled rest period from taking the telomerase activators):
- At the low end of a scheduled rest period, I suggest taking the telomerase activators a minimum of 1 week... before giving them a rest for a few days.
- On the high end of a scheduled rest period, I suggest taking the telomerase activators a maximum of 3 months... before giving them a rest for 1-2 weeks.
Thanks for reminding me Hav,
A
Edited by Anthony_Loera, 26 January 2012 - 03:09 PM.
#1576
Posted 28 January 2012 - 07:27 AM
This was emailed back to me about the question:
I have a question. Is there any reason to believe that extending the telomeres of a cell will have a negative overall health effect? For instance, due to the fact that the internal organelles of the cell have deteriorated from an optimal condition that has nothing to do with telomeres (e.g. mitochondrial disfunction)?
Shorten telomeres are linked to bad health; this has been demonstrated by many studies. Whereas, long telomeres have been closely linked with better health, function and youth. Typically, a cell that has shorten telomeres indicates that the cell has undergone multiple rounds of cell division and is getting old, by then other organelles such as the mitochondria will also begin to malfunction. These events in addition to other cell changes will occur together as the normal process of aging. However, they sometimes occur at slightly different stages. Think of an old car. The engine starts to leak oil and the tires get worn out. The oil leak may be more serious in the long-term. It you can prevent your engine from leaking oil you just might get a few extra miles out of the car. Keeping long telomere is key for a cell’s longevity. So keeping long telomeres is most likely to give our cells a few extra miles. In some cells (such as fibroblasts) being able to keep your telomere intact maintains the rest of the cell’s organelles working properly apparently endlessly.
Hector Valenzuela, Ph.D.
Chief Science Officer
RevGenetics
Does that mean that we can expect mitochondrial disfunction after a certain number of cell divisions, regardless of the length of the telomeres of the cells?
#1577
Posted 28 January 2012 - 02:03 PM
A
#1578
Posted 28 January 2012 - 10:33 PM
Was that a question for Dr. Valenzuela?
A
Yes, if you wouldn't mind.
#1579
Posted 29 January 2012 - 01:10 AM
#1580
Posted 30 January 2012 - 01:15 AM
This was emailed back to me about the question:
I have a question. Is there any reason to believe that extending the telomeres of a cell will have a negative overall health effect? For instance, due to the fact that the internal organelles of the cell have deteriorated from an optimal condition that has nothing to do with telomeres (e.g. mitochondrial disfunction)?
Shorten telomeres are linked to bad health; this has been demonstrated by many studies. Whereas, long telomeres have been closely linked with better health, function and youth. Typically, a cell that has shorten telomeres indicates that the cell has undergone multiple rounds of cell division and is getting old, by then other organelles such as the mitochondria will also begin to malfunction. These events in addition to other cell changes will occur together as the normal process of aging. However, they sometimes occur at slightly different stages. Think of an old car. The engine starts to leak oil and the tires get worn out. The oil leak may be more serious in the long-term. It you can prevent your engine from leaking oil you just might get a few extra miles out of the car. Keeping long telomere is key for a cell’s longevity. So keeping long telomeres is most likely to give our cells a few extra miles. In some cells (such as fibroblasts) being able to keep your telomere intact maintains the rest of the cell’s organelles working properly apparently endlessly.
Hector Valenzuela, Ph.D.
Chief Science Officer
RevGenetics
Does that mean that we can expect mitochondrial disfunction after a certain number of cell divisions, regardless of the length of the telomeres of the cells?
That is correct. Our cell's mitochondria will get some damage that will lead to disfunction regardless of telomere length. I should clarify that not all cells types suffer from mitochondria damage to the same degree. There may be many reasons for this, one reason may be based on the fact that different cells have different amounts of mitochondria. For example muscle fibers (muscle cells) have many mitochondria, whereas red blood cells do not have any mitochondria.
I hope I have answered all your questions. Please don't hesitate to ask if you have anymore.
Hector Valenzuela, Ph.D.
Chief Science Officer
RevGenetics
#1581
Posted 30 January 2012 - 01:45 AM
Answered below:
This was emailed back to me about the question:
I have a question. Is there any reason to believe that extending the telomeres of a cell will have a negative overall health effect? For instance, due to the fact that the internal organelles of the cell have deteriorated from an optimal condition that has nothing to do with telomeres (e.g. mitochondrial disfunction)?
Shorten telomeres are linked to bad health; this has been demonstrated by many studies. Whereas, long telomeres have been closely linked with better health, function and youth. Typically, a cell that has shorten telomeres indicates that the cell has undergone multiple rounds of cell division and is getting old, by then other organelles such as the mitochondria will also begin to malfunction. These events in addition to other cell changes will occur together as the normal process of aging. However, they sometimes occur at slightly different stages. Think of an old car. The engine starts to leak oil and the tires get worn out. The oil leak may be more serious in the long-term. It you can prevent your engine from leaking oil you just might get a few extra miles out of the car. Keeping long telomere is key for a cell’s longevity. So keeping long telomeres is most likely to give our cells a few extra miles. In some cells (such as fibroblasts) being able to keep your telomere intact maintains the rest of the cell’s organelles working properly apparently endlessly.
Hector Valenzuela, Ph.D.
Chief Science Officer
RevGenetics
Does that mean that we can expect mitochondrial disfunction after a certain number of cell divisions, regardless of the length of the telomeres of the cells?
That is correct. Our cell's mitochondria will get some damage that will lead to disfunction regardless of telomere length. I should clarify that not all cells types suffer from mitochondria damage to the same degree. There may be many reasons for this, one reason may be based on the fact that different cells have different amounts of mitochondria. For example muscle fibers (muscle cells) have many mitochondria, whereas red blood cells do not have any mitochondria.
I hope I have answered all your questions. Please don't hesitate to ask if you have anymore.
Hector Valenzuela, Ph.D.
Chief Science Officer
RevGenetics
There's strong evidence that this is in fact NOT true when hTERT is permanently turned on and telomeres are kept permanently long. When a viral vector is used to permanently turn on hTERT in various cell lines, the cells continue to divide without the cell line showing any significant signs of mitochondrial damage. In other words, for example, the cell produced by the 10,000th division shows no more significant mitochondrial damage than the very first cell in the lineage. This was demonstrated in a series of elegant expriements at Geron a decade ago, and reproduced elsewhere multiple times since then.
For in vivo evidence that this in fact works, you need look no further than your own germ cells: Germ cells which naturally have hTERT permanently turned on do not show any significant signs of mitochondrial damage no matter how many times they divide. If they did, your children would be born with damaged mitochondria. That's because your child's first cells are derived directly from your own germ cells by ordinary cell division. But your child is (in most cases) born with perfectly healthy mitochondria, not mitochondria that have suffered accumulated damage as a result of many many cell divisions. Any accumulated mitochondrial damage that may have occurred in the germ cell line before the baby is conceived is at some point in the process completely corrected by mechanisms naturally present in the germ cells themselves.
It's not that the damage doesn't occur when hTERT is turned on permanently. It's that the cells in this case are capable of activating their own internal mechanisms to protect against and repair the damage.
Whether this will happen in all cells in vivo if hTERT is turned on is extremely controversial. But it certainly does happen when hTERT is permanently turned on in a variety of cells in vitro. And it certainly happens in your germ cells.
Edited by Louis, 30 January 2012 - 01:49 AM.
#1582
Posted 30 January 2012 - 01:55 AM
What about the greatly increased risk of all kinds of birth defects after perspective parents (mother and/or father) exceed roughly the age of 40 and those risks exponentially rising for every year past 40? And probably where all the anecdotal evidence over the years about first born came from.Answered below:
This was emailed back to me about the question:
I have a question. Is there any reason to believe that extending the telomeres of a cell will have a negative overall health effect? For instance, due to the fact that the internal organelles of the cell have deteriorated from an optimal condition that has nothing to do with telomeres (e.g. mitochondrial disfunction)?
Shorten telomeres are linked to bad health; this has been demonstrated by many studies. Whereas, long telomeres have been closely linked with better health, function and youth. Typically, a cell that has shorten telomeres indicates that the cell has undergone multiple rounds of cell division and is getting old, by then other organelles such as the mitochondria will also begin to malfunction. These events in addition to other cell changes will occur together as the normal process of aging. However, they sometimes occur at slightly different stages. Think of an old car. The engine starts to leak oil and the tires get worn out. The oil leak may be more serious in the long-term. It you can prevent your engine from leaking oil you just might get a few extra miles out of the car. Keeping long telomere is key for a cell’s longevity. So keeping long telomeres is most likely to give our cells a few extra miles. In some cells (such as fibroblasts) being able to keep your telomere intact maintains the rest of the cell’s organelles working properly apparently endlessly.
Hector Valenzuela, Ph.D.
Chief Science Officer
RevGenetics
Does that mean that we can expect mitochondrial disfunction after a certain number of cell divisions, regardless of the length of the telomeres of the cells?
That is correct. Our cell's mitochondria will get some damage that will lead to disfunction regardless of telomere length. I should clarify that not all cells types suffer from mitochondria damage to the same degree. There may be many reasons for this, one reason may be based on the fact that different cells have different amounts of mitochondria. For example muscle fibers (muscle cells) have many mitochondria, whereas red blood cells do not have any mitochondria.
I hope I have answered all your questions. Please don't hesitate to ask if you have anymore.
Hector Valenzuela, Ph.D.
Chief Science Officer
RevGenetics
There's evidence that this is in fact NOT true when hTERT is permanently turned on. When a viral vector is used to permanently turn on hTERT in various cell lines, the cells continue to divide without the cell line showing any significant signs of mitochondrial damage. In other words, for example, the cell produced by the 10,000th division shows no more significant mitochondrial damage than the very first cell in the lineage. This was demonstrated in a series of elegant expriements at Geron a decade ago, and reproduced elsewhere multiple times since then.
For in vivo evidence that this in fact works, you need look no further than your own germ cells: Germ cells which naturally have hTERT permanently turned on do not show any significant signs of mitochondrial damage no matter how many times they divide. If they did, your children would be born with damaged mitochondria. That's because your child's first cells are derived directly from your own germ cells by ordinary cell division. But your child is (in most cases) born with perfectly healthy mitochondria, not mitochondria that have suffered accumulated damage as a result of many many cell divisions. Any accumulated mitochondrial damage that may have occurred in the germ cell line before the baby is conceived is at some point in the process completely corrected by mechanisms naturally present in the germ cells themselves.
It's not that the damage doesn't occur when hTERT is turned on permanently. It's that the cells in this case are capable of activating their own internal mechanisms to protect against and repair the damage.
Whether this will happen in all cells in vivo if hTERT is turned on is extremely controversial. But it certainly does happen when hTERT is permanently turned on in a variety of cells in vitro. And it certainly happens in your germ cells.
#1583
Posted 30 January 2012 - 02:03 AM
And cells with hTERT permanently turned on in a petri dish also aquire random mutations over time as they divide. The longer they are left dividing for, the more mutations they accumulate. The bad mutations will be selected against and the cells will die. The good mutations will survice. Evolution in action.
#1584
Posted 30 January 2012 - 02:13 AM
That's exactly how evolution works. There's an in-born mechanism that does allow mutations to occur in the germline so the adaptation and natural selection process can occur over many generations. But excluding random mutations that occur as part of this evolutionary process (and yes some of these mutations do occur in the mitochondrial DNA as well), the cell line is completely reset in the fetus. This reset process includes the mitochondria in the cell line.
And cells with hTERT permanently turned on in a petri dish also aquire random mutations over time as they divide. The longer they are left dividing for, the more mutations they accumulate. The bad mutations will be selected against and the cells will die. The good mutations will survice. Evolution in action.
I'm not aware of any good birth defects. And as you pointed out, the greater the age of the parents, the greater the chance of mutations including mitochondrial mutations. And these mutations are never good....as said, never heard of a favorable birth defect. You seem to be contradicting yourself. Can't have your cake and eat it too.
#1585
Posted 30 January 2012 - 02:26 AM
You never heard of a good "birth defect", because the term "birth defect" is a synonym for a "bad mutation".
A whole series of good mutations occured in the germ line over hundreds of thousands of years to allow humans to evolve brains much larger than all other mammmals. Those are all examples of good mutations.
The point is that the germ line is shared between these early small brained mammals and humans today, yet our mitochondria are just as healthy and undamaged as those in mammals from hundreds of thousands of years ago. The germ line has been dividing over and over again for hundreds of thousands of years and the mitochondria remain robust and healthy. But yes there were many random changes due to mutations that occurred in the process and were naturally selected for, which ultimately changed the germline (and the germline mitochondria) for the better.
Edited by Louis, 30 January 2012 - 02:27 AM.
#1586
Posted 30 January 2012 - 02:33 AM
Of course some mutations are good. Otherwise evolution could not occur.
You never heard of a good "birth defect", because the term "birth defect" is a synonym for a "bad mutation".
A whole series of good mutations occured in the germ line over hundreds of thousands of years to allow humans to evolve brains much larger than all other mammmals. Those are all examples of good mutations.
The point is that the germ line is shared between these early small brained mammals and humans today, yet our mitochondria are just as healthy and undamaged as those in mammals from hundreds of thousands of years ago. The germ line has been dividing over and over again for hundreds of thousands of years and the mitochondria remain robust and healthy. But yes there were many random changes due to mutations that occurred in the process and were naturally selected for, which ultimately changed the germline (and the germline mitochondria) for the better.
You're missing the point. The bottom line is that the older the parents, the higher the likelihood of germ mitochondrial damage. You said that yourself. While at the same time, said that germ mitochondrial damage doesn't occur. Which is it? And as far as good or bad mutations...it's like throwing the dice and winning the lottery....for every good mutation you win....you'll lose a million bad mutations...it's the luck of the draw. And as said, the older the parents, the bigger chance of coming up bad, plain and simple.
edit: the point is that the germ does get more mitochondrial mutations as the parent (and the germ) age....and those mutations in aging parents are rarely good.
Edited by Hebbeh, 30 January 2012 - 02:37 AM.
#1587
Posted 30 January 2012 - 03:05 AM
Any accumulated cell damage in the germ line is repaired by the cells themselves before the germ line differentiates into the fetus. The point is that the mechansms are there inside the cell to repair the mitochondrial damage.
If you're asking me why the germ line doesn't repair every single tiny bit of damage that occurs before the fetus is born, I can only speculate: First, every single tiny bit of damage is impossible to repair. Second, it allows an intentional means for random mutation to enter into the gene pool. Evolution will intentionally select for mechanisms that allow randomness to enter the gene pool because it gives a species the ability to adapt and evolve over time, which ensures the continued survival of the species. If there was no intentional mechanism to allow the germ line to mutate randomly, it would die off because the organisms it inhabits would not be able to adapt to changes in their environment over time.
The in vitro experiments back this up: If you permanently turn on hTERT in cells in petri dish, the cells will divide for years and years with no significant signs of mitochondrial damage, i.e. the mitochondria in the 100,000th division will have no more significant damage than the mitochondria in the first cell in the lineage. But yes there will be a small amount of random mutations that occur over time -- that's how nature designed the evolutionary system to work.
Edited by Louis, 30 January 2012 - 03:09 AM.
#1588
Posted 30 January 2012 - 03:24 AM
Like I said before, it's not that this damage doesn't ocurr. It's that the cell posesses the ability to repair it when hTERT is turned on. That's why the fetus is born with robust healthy mitochondria. All the damage is repaired, minus the random mutations that occur.
Any accumulated cell damage in the germ line is repaired by the cells themselves before the germ line differentiates into the fetus. The point is that the mechansms are there inside the cell to repair the mitochondrial damage.
If you're asking me why the germ line doesn't repair every single tiny bit of damage that occurs before the fetus is born, I can only speculate: First, every single tiny bit of damage is impossible to repair. Second, it allows an intentional means for random mutation to enter into the gene pool. Evolution will intentionally select for mechanisms that allow randomness to enter the gene pool because it gives a species the ability to adapt and evolve over time, which ensures the continued survival of the species. If there was no intentional mechanism to allow the germ line to mutate randomly, it would die off because the organisms it inhabits would not be able to adapt to changes in their environment over time.
The in vitro experiments back this up: If you permanently turn on hTERT in cells in petri dish, the cells will divide for years and years with no significant signs of mitochondrial damage, i.e. the mitochondria in the 100,000th division will have no more significant damage than the mitochondria in the first cell in the lineage. But yes there will be a small amount of random mutations that occur over time -- that's how nature designed the evolutionary system to work.
In your original reply, you inferred that germ cells don't incur mitochondrial damage...not whether the cell could or could not repair such damage. And my point is that the older the parent and the germ cells, the higher likelihood that such damage will occur and the higher likelihood that the cell will not be able to repair any damage...but bottom line...nature has cruelly shown us that germ cells are not immune from mitochondrial damage especially as they age. What happens in a petri dish is a moot point in the real world.
For in vivo evidence that this in fact works, you need look no further than your own germ cells: Germ cells which naturally have hTERT permanently turned on do not show any significant signs of mitochondrial damage no matter how many times they divide. Whether this will happen in all cells in vivo if hTERT is turned on is extremely controversial. But it certainly does happen when hTERT is permanently turned on in a variety of cells in vitro. And it certainly happens in your germ cells.
As discussed...and as natures as shown us...this is simply not true.
#1589
Posted 30 January 2012 - 03:31 AM
#1590
Posted 30 January 2012 - 03:43 AM
If it were not true, your children would be born with damaged mitochrondia. The germ line resets any minor damage with each new generation and the mitochondria remain pristine.
You purposely keep dancing in circles and changing your position. The issue is not whether germ cells can repair damage or not. The original issue, as I reminded you through your original quote above, is whether germ cells experience mitochondrial damage at all. Do I need to repost your quote again? I will remind you that your original post claimed germ cells are immune to mitochondrial damage. That they will not experience any mitochondrial damage no matter how many times they divide. Are you sticking to this claim? Or have you changed your mind?
edit: hint....if germ cells didn't experience mitochondrial damage...they wouldn't have to reset or repair said damage.
double edit: And my original point was that the older the germ cell, the higher likelihood of mutations as evidenced by the exponentially higher rate of birth defects in aging parents. Which would seem to indicate that germ cells show increasing damage as they age just like other cells.
Edited by Hebbeh, 30 January 2012 - 04:02 AM.
5 user(s) are reading this topic
0 members, 5 guests, 0 anonymous users






























 This topic is locked
This topic is locked