The research community has for some years now seemed on the verge of making real progress in the elimination of tooth decay and periodontal disease. That final leap from understanding and promising research to earnest clinical development always takes longer than we'd like it to, however. The most pressing and widespread dental issues are largely bacterial in origin, but not quite as straightforward as simply identifying one unwanted type of microbe and getting rid of it. This is a story of interactions between the behavior of different species, and the need to eliminate harmful behavior without disrupting the activities of the many beneficial bacterial species found in the mouth. The work needed to draw closer to practical treatments has developed across the course of this decade.
We should care about the decay of the mouth for the same reason we should care about the decay of the rest of the body. Every piece of our physiology is useful in some way. Further, everything is connected, and the harms that bacteria cause to gums in particular results in inflammation that spreads into other tissues. There is a strong association between the presence of forms of oral bacteria known to be problematic and overall mortality rates in later life. Given what we know of the role of inflammation in aging, this should not be surprising: it accelerates the development and progression of all of the common age-related diseases.
As the research noted here illustrates, the mouth is a proving ground for a range of approaches to the targeted sabotage of bacteria: efforts to remove specific bad behavior while changing as little else as possible. This strategy is almost forced on the medical community by necessity. The mouth is about as far from a sterile environment as it is possible to get, one in which even sophisticated attempts to eliminate bacteria for the long term usually prove futile. It is also home to many useful bacterial species whose removal will only cause issues, even if it was practical to keep them out for longer than a few days or weeks. The sort of heavy-handed antibacterial strategies that work so well for infections elsewhere in the body, or to eliminate bacterial strains that are only rarely encountered, and will not immediately replenished from the environment, are not useful here. Success in the development of more targeted approaches to oral bacteria may well find use elsewhere, however.
Small molecule inhibitor prevents or impedes tooth cavities in a preclinical model
Researchers have created a small molecule that prevents or impedes tooth cavities in a preclinical model. The inhibitor blocks the function of a key virulence enzyme in an oral bacterium, a molecular sabotage that is akin to throwing a monkey wrench into machinery to jam the gears. In the presence of the molecule, Streptococcus mutans - the prime bacterial cause of the tooth decay called dental caries - is unable to make the protective and sticky biofilm that allows it to glue to the tooth surface, where it eats away tooth enamel by producing lactic acid. This selective inhibition of the sticky biofilm appears to act specifically against S. mutans, and the inhibitor drastically reduced dental caries in rats fed a caries-promoting diet. "Our compound is drug-like, non-bactericidal and easy to synthesize, and it exhibits very potent efficacy in vivo. It is an excellent candidate that can be developed into therapeutic drugs that prevent and treat dental caries."
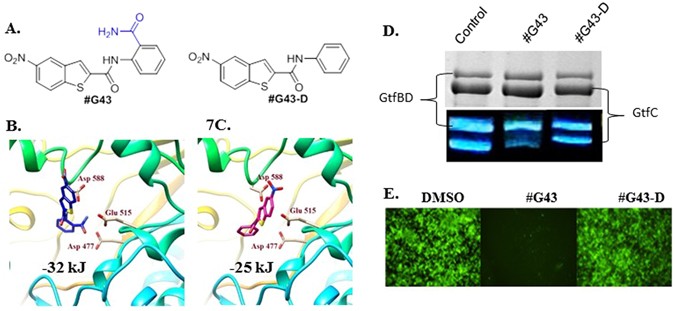
The glucan biofilm is made by three S. mutans glucosyltransferase, or Gtf, enzymes. The crystal structure of the GtfC glucosyltransferase is known, and the researchers used that structure to screen - via computer simulations - 500,000 drug-like compounds for binding at the enzyme's active site. Ninety compounds with diverse scaffolds showing promise in the computer screening were tested for their ability to block biofilm formation by S. mutans in culture. Seven showed potent inhibition and one, #G43, was tested more extensively. #G43 inhibited the activity of enzymes GtfB and GtfC. #G43 did not inhibit the expression of the gtfC gene, and it did not affect growth or viability of S. mutans and several other oral bacteria tested. Also, #G43 did not inhibit biofilm production by several other oral streptococcal species. In the rat-model of dental caries, animals on a low-sucrose diet were infected with S. mutans and their teeth were treated topically with #G43 twice a day for four weeks. The #G43 treatment caused very significant reductions in enamel and dentinal caries.
Structure-Based Discovery of Small Molecule Inhibitors of Cariogenic Virulence
Dental caries is a multifactorial disease of bacterial origin, which is characterized by the localized destruction of dental hard tissues. Though the oral cavity harbors over 700 different bacterial species, Streptococcus mutans initiates the cariogenic process and remains as the key etiological agent. Using key matrix producing enzymes, glucosyltransferases (Gtfs), S. mutans produces sticky glucosyl glucan polymers, which facilitate the attachment of the bacteria to the tooth surface. The glucans is a major component of the biofilm matrix that shields the microbial community from host defenses. Furthermore, copious amounts of lactic acid are produced as a byproduct of bacterial consumption of dietary sugars within the mature biofilm community, which ultimately leads to demineralization of the tooth surface, ensuing cariogenesis.
Selectively targeting cariogenic pathogens such as S. mutans has been explored previously, however it was found that the antimicrobial peptide also alters the overall microbiota. Our increasing understanding of bacterial virulence mechanisms provides new opportunities to target and interfere with crucial virulence factors such as Gtfs. This approach has the added advantages of not only being selective, but may also help to preserve the natural microbial flora of the mouth, which may avoid to exert the strong pressure to promote the development of antibiotic resistance, overcoming a major public health issue in the antibiotic era. It is well established that glucans produced by S. mutans Gtfs contribute significantly to the cariogenicity of dental biofilms. Therefore, the inhibition of the Gtf activity and the consequential glucan synthesis would impair the S. mutans virulence, which could offer an alternative strategy to prevent and treat biofilm-related diseases.
S. mutans harbors three Gtfs: GtfB, GtfC, and GtfD. Previous studies have demonstrated that glucans produced by GtfB and GtfC are essential for the assembly of the S. mutans biofilms. We conducted an in silico screening of 500,000 drug-like small molecule compounds targeting GtfC and identified top scored scaffolds for in vitro biofilm assays. Seven potent biofilm inhibitors emerged from this study, the lead compound #G43 was further characterized and shown to have anti-biofilm activity through the binding to GtfBC and the inhibition of the activity of GtfBC. The lead compound drastically reduced bacterial virulence in a rat model of dental caries.
View the full article at FightAging