Crosslinks
Oct 07 2011 12:48 PM |
ImmInst
in Articles
 by Johan Sjöberg
by Johan Sjöberg The diseases and complications of aging are infamous –cardiovascular disease, cataracts, Alzheimer's disease, amyloidosis, cancer, and so on. Their effects are often debilitating, and one of them will always end up causing your death in the end. Until relatively recently, though, not much was known about exactly why these things happen. Only since the advent of modern biochemistry and biomedicine have we been able to start exploring the aging process in all its intricate details. However, the world of cellular aging turns out to be far more complex than we initially thought. Aging is, at its most fundamental level, based on genes and proteins. For example, cells have a finite capacity to divide, and this is thought to be controlled by the length of the ends of our DNA, called telomeres. They act as protective caps, preventing degradation of genes that are important to maintain cellular function. With each cell division, the telomeres are shortened, and when they reach a certain length, the cell normally doesn't divide any further. This may cause various tissues of the body to start to degenerate or diminish, since there are not enough cells produced to maintain them. Alternatively, mutations may occur that allow the cell to continue dividing uncontrollably, inevitably leading to cancer. Further examples are that the proteins and components of the cell cannot break down certain cellular waste-products, causing them to accumulate and potentially become damaging to the body, or that the proteins themselves are modified so that they lose their function or stick together. Alternatively, errors in cellular metabolism may cause reactions in undesired ways, such as oxygen reacting with and damaging various cellular components, or glucose linking together proteins in deleterious ways. There are many other such examples that I won't list here, but they are all associated with the diseases of aging in some way. For example, Alzheimer's disease is caused by the degeneration of nerve cells in the brain, and the undesired linking of proteins that are found in the walls of blood vessels may make them less elastic, leading to higher blood pressure and an increased risk of cardiovascular disease.  In order to do something about aging and its associated diseases, then, one might intuitively want to start at the level of genes and proteins. If one can protect the genes or the proteins they encode from damage, enhance their function in some way to make them more efficient, or introduce new genes to deal with problems that can't be dealt with naturally, one might prevent the complications of aging from even occurring in the first place. However, the genetics of aging and cellular metabolism is extremely complex, and multiple genes and proteins are often dependent on each other. If one gene is modified, this may affect many others, leading to unforeseen and potentially undesirable consequences. On the other end of the spectrum of interventions in aging is geriatric medicine, which focuses on treating the symptoms of the diseases of aging. Unfortunately, the damage has already been done by then, and trying to completely reverse diseases of aging which have already reached a pronounced stage has so far not proven to be successful. However, there might be a third way, a middle ground of sorts – attempting to prevent or remove the accumulated waste-products and the results of errors in metabolism, before their levels become high enough to cause diseases. This is the main pathway proposed by Dr. Aubrey de Grey and the SENS Foundation, a non-profit organisation that is aimed at the development of rejuvenation technologies that address the complications of aging. One example of such accumulation is the linking of proteins, or cross-linking, that I mentioned earlier, and that we will now explore in more detail. Cross-linking is the result of a substance, most often some kind of sugar, reacting with two or more protein molecules and linking them together. The effects of cross-linking is perhaps most significantly seen in collagen, which is the most common protein in the human body. Collagen is a major component of bone, tendons, blood vessel walls and skin, among other things, where it adds strength, support and elasticity by being an important part of the supportive structure, called the extracellular matrix, that surrounds our cells on the outside. In many ways, the matrix is similar to rubber, being both strong and flexible at the same time. However, also like rubber, the matrix loses its strength and flexibility with age, and this is where cross-linking comes into play. Collagen is made of long threads or fibers, and these move in relation to each other when the collagen is stretched, like for instance when you pinch your skin, or when a blood vessel is widened as blood is pumped through it. When the collagen fibers are linked together by cross-links, the fibers cannot move as much as before, and the collagen becomes more rigid. Besides collagen, there are several other important proteins in the matrix, and they too have their specific roles – for example, there are special proteins that tie the collagen fibers to the outsides of the cells that the matrix supports. That's not to say that there should be no cross-linking at all, though; on the contrary, a certain amount of cross-links in the matrix are created in a controlled way by the body to keep it together. This is a vital process for normal body function. However, it is the excess cross-linking that occurs uncontrollably, at random places on proteins in the matrix, that is responsible for the age-related damage that is associated with cross-linking [1]. As I mentioned earlier, the substance that causes the cross-linking is often some kind of sugar, such as glucose or fructose. Since almost all of the sugar in the body exists in the blood stream, the collagen in blood vessel walls, skin, and other tissues that are exposed to a steady stream of blood has the highest rate of uncontrolled cross-linking. The cross-linking process occurs very slowly and in several steps, the most critical of which is the formation of a substance called an advanced glycation endproduct ("AGE" for short – great abbreviation, right?). There are many different kinds of AGEs, which may react to form cross-links more or less easily. However, once the AGE is formed and linked to a protein, the body cannot cleave it off, and has to replace the protein itself in order to get rid of the AGE. In long-lived proteins such as collagen, which has a life-span of many years, that gives the AGE more than enough time to react with a second collagen fiber and form a cross-link between the two. In collagen, the most common cross-link is called glucosepane [2-3] and is formed from glucose, or blood sugar, but there are several others as well. AGEs can form both inside and outside of the cells. On the inside, they modify the cells' proteins, which makes them less able to do their job, and this disrupts the cells' metabolic processes. On the outside, it makes the ECM less flexible. 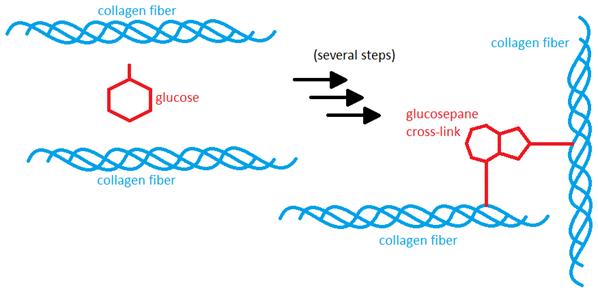 Since glucose is occasionally able to react with proteins and form cross-links in this way, hyperglycemia and diabetes lead to more cross-linking than in people with normal blood glucose levels. In one study, researchers found that the levels of the most common collagen cross-link, glucosepane, was more than twice as high in diabetics compared to healthy people. This cross-linking may be responsible for some of the complications sometimes seen with diabetes, as well as with normal aging. One example is in the kidneys, where a part of the kidney called the glomerular basement membrane is crucial for proper function, since it is the part that performs the actual filtration of small substances from the blood. The membrane contains a lot of collagen, and excess cross-linking causes this collagen to clump together and act less like a web, thereby making the pores in the membrane larger and allowing more and larger molecules, including whole proteins, to escape from the bloodstream, thus greatly impairing its function as a tight filter. Diabetics run a much higher risk of kidney failure, partly because of excess collagen cross-linking, since higher glucose levels allow for more cross-links to form [2]. Another example of the effects of excess cross-linking can be found in the lens of the eye. The lens is made up of proteins called crystallins, which provide the lens with rigidity and flexibility while still allowing it to remain transparent. However, like many other proteins, these crystallins can also be cross-linked, and this causes them to clump together in much the same way as the collagen in the kidney. The effect of this is that the lens may become more cloudy, impairing vision and possibly leading to the formation of cataracts [4]. A third example is the collagen in the walls of blood vessels,which may have an increased amount of cross-linking with aging. This makes the blood vessel walls stiffer and therefore less able to expand when blood is pumped through the vessel, leading to higher blood pressure [5]. Again, diabetes increases the risk for both these things. You may recall my mentioning that controlled cross-linking is vital for proper functioning of the extracellular matrix. There are a few ways for the body to make these cross-links, and they all involve special enzymes (proteins) that are very efficient in forming them, as well as other enzymes that are able to break the cross-links. However, even these cross-links have the potential of causing problems if they become too numerous. If the production of the enzyme that makes the cross-links increases beyond normal levels, or if the activity of each enzyme molecule is increased, more cross-links will be created than are normally present. This over-abundance of cross-links may contribute to the same problems as the cross-links that are created randomly by, for example, glucose [6].  The formation of AGEs is not confined to the body itself; AGEs can also form outside of the body, in the process of cooking and heating food, by cooking sugars and proteins together. For example, the characteristic browning of grilled meat or baked bread is an indication that AGEs have been formed. (The first steps of this reaction was discovered in 1912 by Louis-Camille Maillard, and is therefore named the Maillard reaction.) The reaction is enhanced by high temperatures, low moisture, and long cooking times, making boiling one of the cooking methods generating the least amount of AGEs, and frying one of the methods generating the most AGEs. Adding sugar to heated food also facilitates AGE formation, since there will be more molecules around that can form cross-links between proteins in the food items. Various meats typically have more AGEs in them than other kinds of foods, too, especially when they are fried or barbecued. The formation of AGEs is not confined to the body itself; AGEs can also form outside of the body, in the process of cooking and heating food, by cooking sugars and proteins together. For example, the characteristic browning of grilled meat or baked bread is an indication that AGEs have been formed. (The first steps of this reaction was discovered in 1912 by Louis-Camille Maillard, and is therefore named the Maillard reaction.) The reaction is enhanced by high temperatures, low moisture, and long cooking times, making boiling one of the cooking methods generating the least amount of AGEs, and frying one of the methods generating the most AGEs. Adding sugar to heated food also facilitates AGE formation, since there will be more molecules around that can form cross-links between proteins in the food items. Various meats typically have more AGEs in them than other kinds of foods, too, especially when they are fried or barbecued.(In fact, according to one study [7], a typical fast-food hamburger contains as much AGEs as about 25 kg of canned corn, or 100 liters of whole milk!) The AGEs that are created in cooked food can be absorbed by the body, and once they have been absorbed, they can bind to certain receptors called RAGE (Receptor for AGE), and this may contribute to some chronic kidney diseases [8], as well as chronic inflammatory diseases such as atherosclerosis [9]. Presumably, these AGEs may also be able to contribute to protein cross-linking in the same way as AGEs that are created within the body. Thus, there are two different ways that AGEs can contribute to age-related complications –cross-linking and inflammation. But despite the complexity of AGEs and cross-linking, there is hope on the horizon. Developing drugs that can break cross-links may be possible with current technology, and research is already ongoing or planned at various locations, including a possible project (which I hope to be a part of) funded by the SENS Foundation at Cambridge University, aimed at finding a way to break the most common collagen cross-link, glucosepane. Another way to reduce excess cross-linking with aging is to prevent cross-link formation, and in that area, there has been more success. Several compounds that may prevent excess cross-link formation to some degree are available as dietary supplements; two examples are carnosine [10] and benfotiamine [11]. In addition, there are a number of things you can do yourself to reduce the amount of excess cross-linking in your own body; keeping blood sugar levels under control by avoiding too much sugars and simple carbohydrates is one thing, and reducing the consumption of grilled meat could be another. So, in conclusion, science is advancing to deal with the problems of excess cross-linking, although we're not quite there yet. And cross-linking is only a small part of the aging process. However, there is good progress in other areas of aging research as well. Meanwhile, if you take good care of your health, you will increase your chances of living to see the day when definitive treatments for the diseases of aging are finally available, allowing you to live a much longer life in good health. And, to be honest, who doesn't want that? References for further reading extracellular matrix. Relationship with diabetes. J Biol Chem. 2005 Apr 1; 280 (13). 12310-5. pathophysiology of glucosepane: a major protein cross-link. Rejuvenation Res. 2009 Apr; 12 (2): 137-48. modifications in lens aging and cataract formation. Invets Ophthalmol Vis Sci. 1998 Nov; 39 (12): 2355-64. S-nitrosylation of tissue transglutaminase contributes to age-related increases in vascular stiffness. Circ Res. 2010 Jul 9; 107 (1): 117-25. Efficacy of benfotiamine versus thiamine on function and glycation products of peripheral nerves in diabetic rats. Exp Clin Endocrinol Diabetes. 2001; 109 (6): 330-6. |
Search Articles |
-


 Sign In
Sign InGoogle Sign in options
- Create Account








































1 Comments