.
S O U R C E : Josh Mitteldorf's blog 'Aging Matters'.
Methylation clocks are far and away the most accurate markers of a person’s age, and so are a promising tool for evaluating anti-aging interventions, but they are a bit of a black box. We know from statistics that certain places on chromosomes become steadily methylated (or demethylated) with age, but we often don’t know what effect that has on expression of particular genes.
For the first time, a clock has been devised based on proteins in the blood that is comparable in accuracy to the best methylation clocks. This has the advantage of being downstream of epigenetics, so it is less of a black box. What can we learn from the proteins that are increased (and decreased) with age?
I’ve written often and enthusiastically about the utility of methylation clocks for evaluation of anti-aging interventions [blog, blog, blog, journal article]. This technology offers a way to promptly identify small age-reversal successes (perhaps not in individuals, but averaged over a cohort of ~50 to 100 subjects). Before these tests were available, we had no choice but to wait — usually 10 years or more — for enough experimental subjects to die that we could be sure the intervention we were evaluating affected life expectancy. (This is the plan of the worthy but ridiculously expensive TAME trial promoted by Nir Barzilai.)
Can we rely on methylation clocks to evaluate anti-aging interventions? If we succeed in setting back the methylation clocks, are we actually making the body younger? The answer depends critically on the relationship of methylation to aging.
The majority view derives from the belief that aging is a passive process, while methylation (epigenetics) is a process under tight evolutionary control. The majority holds that methylation changes with age are a response to the damage that accrues unavoidably, and the changes in gene expression that result are actually the body’s best effort to fight back against this damage.
My view is with the minority. Aging is a programmed process (evolved, I believe, for the purpose of demographic stability). Changes in methylation and epigenetic changes generally are the primary cause of aging. Far from being a response to damage, epigenetic changes with age invoke the very signals that cause damage (e.g. inflammation) and simultaneously cut back our repair processes (e.g., detoxification and autophagy).
If you hold with the majority, then setting back the methylation clock (with drugs or gene therapies or …) could actually shorten our lifespans. Setting back the methylation clock means thwarting the body’s efforts to rescue itself. We should not use methylation clocks as a measure of whether a particular technology has achieved rejuvenation.
If you hold with the minority, then setting back the methylation clock is an indication that whatever we have done has struck at the root cause of aging, reversing the epigenetic changes that are the primary driver of senescence.
(In the scientific community of aging, there are a few of us speaking directly about the primary importance of epigenetics [Horvath, Barja, Johnson, Rando, Mitteldorf ], and many more who are tacitly accepting the idea that setting back the methylation clock is a good thing. Most scientists remain skeptical and are not embracing the methylation clocks as a reliable gauge for anti-aging technologies [Han, West].)
The battle lines are not clearly drawn, and the basic conflict in beliefs is not yet out in the open. But resolution of this issue is a major next step for geriatric research. I say this because it is likely there is some truth on each side. Most of the epigenetic changes with age are drivers of senescence (Type 1), but some are the body’s attempts to rescue itself from damage (Type 2). Each of the methylation clocks that are now available averages hundreds of methylation sites, and it is likely that they are a mixture of sites that play these two opposing roles. [background in my October blog]
So the urgent need is for a clock that is constructed exclusively of drivers of aging (Type 1), so that we can use it with confidence as a measure of whether an intervention that we are testing will extend lifespan.
Can we design experiments with the methylation clock that would tell us which of the age-related methylation sites are Type 1 and which are Type 2? It’s hard to know how to begin, because we don’t yet have a way to do controlled experiments. What we want is a molecular tool that will methylate a selected target CpG site while leaving everything else untouched, and we don’t have that yet. (It may become feasible as CRISPR technology improves.) Based on present technology, the only way to tell for sure is to compare how different interventions affect the methylation clocks in thousands of experimental subjects, and then wait and wait and wait and see how long these subjects live. LEF is undertaking this ambitious plan, but it will be decades before it bears fruit.
Clocks based on the proteome
This month, a new clock came out of the Stanford lab of Tony Wyss-Coray that is based on measuring levels of proteins in blood plasma, rather than patterns of methylation on chromosomes. It is not the first proteomic clock, but it is the most accurate. For some of the proteins that feature prominently in the clock, we have a good understanding of their metabolic function, and for the most part they vindicate my belief that epigenetic changes are predominantly drivers of senescence rather than protective responses to damage.
Wyss-Coray was one of the people at Stanford responsible for the modern wave of research in hetrochronic parabiosis. In a series of experiments, they surgically joined a young mouse to an old mouse, such that they shared a blood supply. The old mouse got younger and the young mouse got older, though both suffered early death from their cruel and macabre condition (excuse my editorial license). Later, it was found that chemical constituents of the blood plasma (proteins and RNAs but not whole cells) were responsible for moderating the effective ages of the animals. As part of the current study, Wyss-Coray compared the proteins in the new (human) proteome clock with the proteins that were altered in the (mouse) parabiosis experiments, and found a large overlap. This may be the best evidence we have that the proteome changes are predominantly Type 1, causal factors of senescence. (Here is a very recent BioRxiv preprint of a UCSD study relating epigenetic clocks in people to mice and dogs.)
Different proteins change at different ages
The Stanford group notes that some of the proteins in their clock increase in the blood with age and some decrease. Typically, the changes do not occur uniformly over the lifespan. Though none of the curves is U-shaped (on-off-on, or off-on-off), some proteins do most of their changing early in life, and some later.
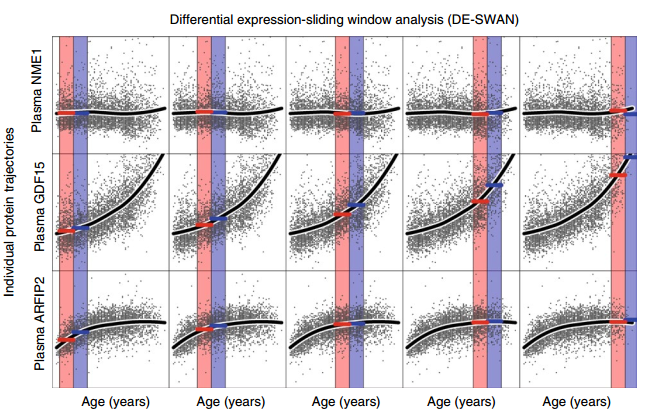
The group identifies three life periods and three groups of proteins: mid-30s, ~60yo, and late 70s.
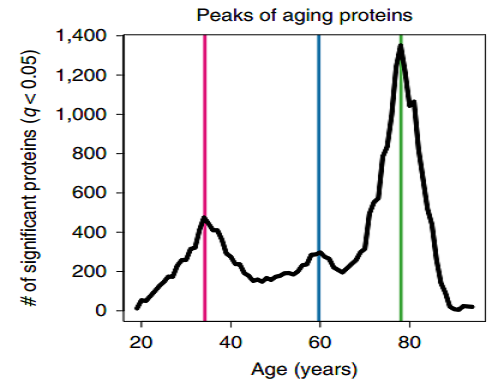
At young age (34 years), we observed a downregulation of proteins involved in structural pathways, such as the extracellular matrix. These changes were reversed in middle and old age (60 and 78 years, respectively). At age 60 years, we found a prominent role of hormonal activity, binding functions and blood pathways. At age 78 years, key processes still included blood pathways but also bone morphogenetic protein signaling, which is involved in numerous cellular functions. Pathways changing with age by linear modeling overlapped most strongly with the crests at age 34 and 60 years (Fig below), indicating that dramatic changes occurring in the elderly might be masked in linear modeling by more subtle changes at earlier ages. Altogether, these results showed that aging is a dynamic, non-linear process characterized by waves of changes in plasma proteins that reflect complex shifts in biological processes.
This paragraph doesn’t tell all we need to know to decide which changes are Type 1 and which Type 2. There is more information in their Supplementary Tables 5 and 14. I don’t have the expertise in biochemistry or metabolics to extract the information, but if you do and you are reading this, I hope you will contact me.
.../...
.
















































