.
C O M P L E T E T I T L E : Pharmacological inhibition of mTOR attenuates replicative cell senescence and improves cellular function via regulating the STAT3-PIM1 axis in human cardiac progenitor cells.
O P E N A C C E S S S O U R C E : nature
Abstract
The mammalian target of rapamycin (mTOR) signaling pathway efficiently regulates the energy state of cells and maintains tissue homeostasis. Dysregulation of the mTOR pathway has been implicated in several human diseases. Rapamycin is a specific inhibitor of mTOR and pharmacological inhibition of mTOR with rapamycin promote cardiac cell generation from the differentiation of mouse and human embryonic stem cells. These studies strongly implicate a role of sustained mTOR activity in the differentiating functions of embryonic stem cells; however, they do not directly address the required effect for sustained mTOR activity in human cardiac progenitor cells. In the present study, we evaluated the effect of mTOR inhibition by rapamycin on the cellular function of human cardiac progenitor cells and discovered that treatment with rapamycin markedly attenuated replicative cell senescence in human cardiac progenitor cells (hCPCs) and promoted their cellular functions. Furthermore, rapamycin not only inhibited mTOR signaling but also influenced signaling pathways, including STAT3 and PIM1, in hCPCs. Therefore, these data reveal a crucial function for rapamycin in senescent hCPCs and provide clinical strategies based on chronic mTOR activity.
Introduction
Aging is associated with an organism progressively losing the ability to maintain homeostasis and repair tissue damage1. The predominant aging mechanism occurs because of the accumulation of senescent cells in various tissues and organs2. These senescent cells have undergone an irreversible cell cycle arrest3 and are characterized by the expression of p16Ink4a4. The prevalence of cardiovascular diseases increases with aging. Investigators have demonstrated that the number of the left ventricle cardiomyocytes progressively declines with aging5. Several studies have reported that undifferentiated primitive cells reside in mammalian hearts and protect them against heart failure1,6,7. Furthermore, senescent and dysfunctional resident human cardiac progenitor cells (hCPCs) accumulate as a consequence of cardiac pathology8,9,10,11,12 and lead to premature cardiac aging and heart failure13. These hCPCs act as key regulators of cardiomyocyte homeostasis in the heart14,15 and contribute to the repair of damaged heart tissue16.
The mammalian target of rapamycin (mTOR) is a serine–threonine protein kinase that regulates vital processes, including protein synthesis, autophagy, cell growth, metabolism, and survival17,18,19,20. mTOR exists in two independent multiprotein-containing complexes (mTORC1 and mTORC2), which have distinct functions in the adult tissue21. mTOR plays an indispensable role in embryonic stem cell maintenance, embryonic heart development, and adult heart homeostasis22,23,24 during cardiogenesis. In addition, mtor knockout mice were shown to exhibit heart failure and dilated cardiomyopathy25,26. Rapamycin is a specific inhibitor of mTOR and is known to be useful in treating diseases such as cancer, diabetes, obesity, neurological diseases, and genetic disorders27. Recent studies demonstrated that rapamycin is an mTORC1 antagonist28,29,30 that can also inhibit mTORC2 activity in some cell types31. The other ATP-competitive inhibitors of mTOR, namely, PP242, have recently been demonstrated to have more potent antileukemic activity than rapamycin32. In addition, rapamycin can efficiently promote cardiac cell generation from the differentiation of mouse embryonic stem cells33,34. These observations indicate that chronic mTOR activity is important for the differentiation of embryonic stem cells into cardiac cells; however, the role of chronic mTOR activity in hCPC regulation remains unclear.
In this study, we demonstrated that mTOR inhibition by rapamycin markedly attenuated replicative cell senescence in hCPCs and promoted cellular functions such as proliferation, migration, clonogenicity, and differentiation. Moreover, rapamycin not only inhibited mTOR signaling but also influenced the STAT3-PIM1 signaling pathway in hCPCs. Collectively, our data reveal the crucial function of rapamycin in senescent hCPCs, which could be important for developing novel therapeutic interventions.
.../...
Results
Characterization of replicative senescent hCPCs
To characterize the phenotype of replicative senescence in hCPCs, we maintained the cells over 16 passages to generate spontaneous replicative senescence (Supplementary Fig. S1). In accordance with previous studies on cellular replicative senescences2,3,4, the length and width of the cells and the SA-β-gal+ activity were increased in senescent hCPCs (Supplementary Fig. S1A, B) compared with that of young controls (less than eight passages). In addition, the expression of both cyclin-dependent kinase inhibitors and senescence-associated markers, such as p21 and p16, was increased in the senescent hCPCs (Supplementary Fig. S1C, D). Furthermore, to address the function of replicative senescence in hCPCs, we evaluated the proliferation, migration, and tube formation abilities of senescent hCPCs (Supplementary Fig. S1E–G). These abilities of the capillary network were significantly reduced in the senescent hCPCs compared with the control of young cells (Supplementary Fig. S1E–G). Furthermore, to determine whether the transcriptome program was altered in the senescent hCPCs, we analyzed their transcripts via RNA-seq (Supplementary Fig. S1H). We found that 2249 and 2420 transcripts were upregulated and downregulated in senescent hCPCs, respectively, including genes that regulate the cell cycle and DNA replication. These data suggest that we initially generated cellular replicative senescence in the hCPCs and characterized the molecular and physiological phenotype of replicative senescence in hCPCs.
Effects of prolonged rapamycin treatment in senescent hCPCs
Several studies have reported that rapamycin efficiently promotes cardiac cell generation from the differentiation of mouse embryonic stem cells33,34. These observations indicate that chronic mTOR activity is important for the differentiation function of embryonic stem cells; however, the role of chronic mTOR activity in senescent hCPCs remains unclear.
Initially, to investigate rapamycin cytotoxicity in senescent hCPCs, we performed MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assays in senescent hCPCs after treating them with different concentrations of rapamycin (Fig. 1a); with 1 µM rapamycin, no significant difference was observed in cell viability in senescent hCPCs. Thus, we selected 100 nM as the concentration of rapamycin for the following experiments (Fig. 1–6). Furthermore, to address the effects of prolonged rapamycin treatment on senescent hCPCs, we continually treated cells with rapamycin during passaging (Fig. 1b). The cell length, SA-β-gal+ activity, and the expression of senescence-associated markers (p53, p21, and p16) were significantly decreased in the senescent hCPCs after prolonged rapamycin treatment (Fig. 1c–f). The major feature of senescent cells is the secretion of cytokines and growth factors, which is termed the senescence-associated secretory phenotype (SASP)36. The expression of genes related to SASP were increased in the senescent hCPCs as shown by column clustered heat maps (Fig. 1g). In these hCPCs, after prolonged rapamycin treatment, the expression of genes related to SASP was decreased to levels similar to those in the young control hCPCs. The expression of SASP components, such as IL1A and IL6, in the senescent hCPCs after prolonged rapamycin treatment was significantly decreased compared with that of the senescent hCPCs (Fig. 1h).
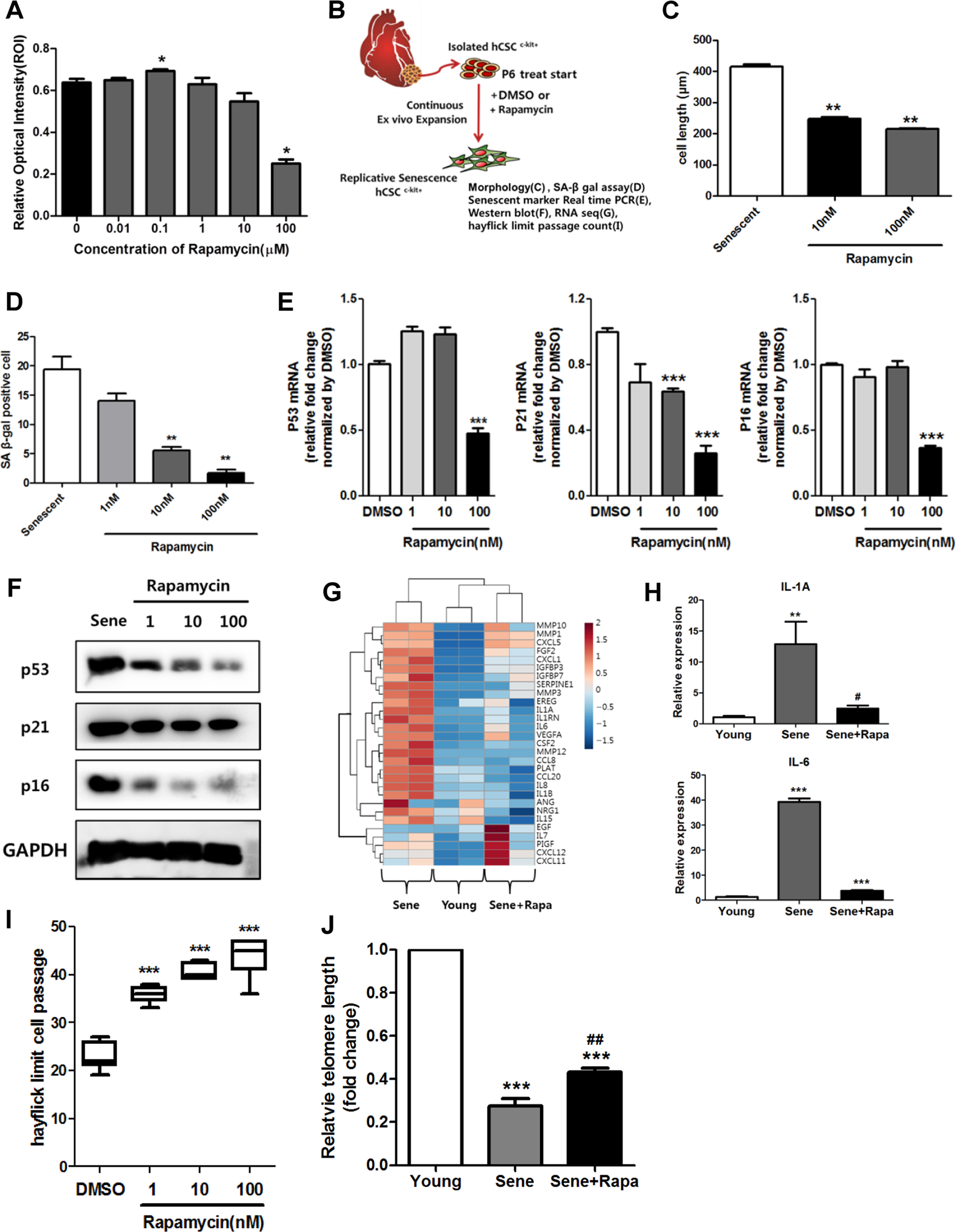
a A cytotoxicity assay was used to assess senescent hCPCs after prolonged rapamycin treatment using an MTS kit (*p ≤ 0.05, n = 8). b Scheme of experiments. c Quantification of the length of senescent hCPCs after prolonged rapamycin treatment (**p ≤ 0.01, n = 10). d Quantification of SA-β-gal staining after prolonged treatment with different concentrations of rapamycin. e, f Measurement of the expression of the following senescent markers: p53, p21, and p16 (***p ≤ 0.001, n = 3). g Heat map of SASP-related genes. h Expression levels of IL1A and IL6 were analyzed in senescent hCPCs after prolonged rapamycin treatment using qRT-PCR (**p ≤ 0.01, n = 3). i A count of the Hayflick passage numbers in the senescent hCPCs after prolonged rapamycin treatment (***p ≤ 0.001, n = 6). j Measurement of the relative telomere length in the senescent hCPCs after prolonged rapamycin treatment (***p ≤ 0.001, vs. young, ## ***p ≤ 0.01, vs. senescent, n = 3). Error bars indicate SEM.
.../...
.










































