.
Abstract
It is hypothesized that treating the general aging population with compounds that slow aging, geroprotectors, could provide many benefits to society, including a reduction of age-related diseases. It is intuitive that such compounds should cause minimal side effects, since they would be distributed to otherwise healthy individuals for extended periods of time. The question therefore emerges of how we should prioritize geroprotectors discovered in model organisms for clinical testing in humans. In other words, which compounds are least likely to cause harm, while still potentially providing benefit? To systematically answer this question we queried the DrugAge database—containing hundreds of known geroprotectors—and cross-referenced this with a recently published repository of compound side effect predictions. In total, 124 geroprotectors were associated to 800 unique side effects. Geroprotectors with high risks of side effects, some even with risk for death, included lamotrigine and minocycline, while compounds with low side effect risks included spermidine and D-glucosamine. Despite their popularity as top geroprotector candidates for humans, sirolimus and metformin harbored greater risks of side effects than many other candidate geroprotectors, sirolimus being the more severe of the two. Furthermore, we found that a correlation existed between maximum lifespan extension in worms and the likelihood of causing a side effect, suggesting that extreme lifespan extension in model organisms should not necessarily be the priority when screening for novel geroprotectors. We discuss the implications of our findings for prioritizing geroprotectors, suggesting spermidine and D-glucosamine for clinical trials in humans.
Introduction
Treating the general aging population with compounds that slow aging, geroprotectors, could provide many benefits to society. Specifically, it is believed that such compounds could reduce the onset of age-related diseases and thereby result in an extension of the healthy years of life. Meanwhile, the identification of novel compounds that extend lifespan in model organisms has been accelerating at an unprecedented rate, as can be visualized by the geroprotector entries from the DrugAge database (Barardo et al. 2017), plotting number of drugs registered per publication date (Fig. 1a). This phenomenon is likely due to a variety of factors, including the increased use of short lived model organisms such as Caenorhabditis elegans (worms), the invention and implementation of higher throughput technologies to assess lifespans, and computational drug screening approaches helping to identify novel geroprotectors (Stroustrup et al. 2013; Carretero et al. 2015; Janssens et al. 2019; Calvert et al. 2016; Petrascheck et al. 2007; Ye et al. 2014).
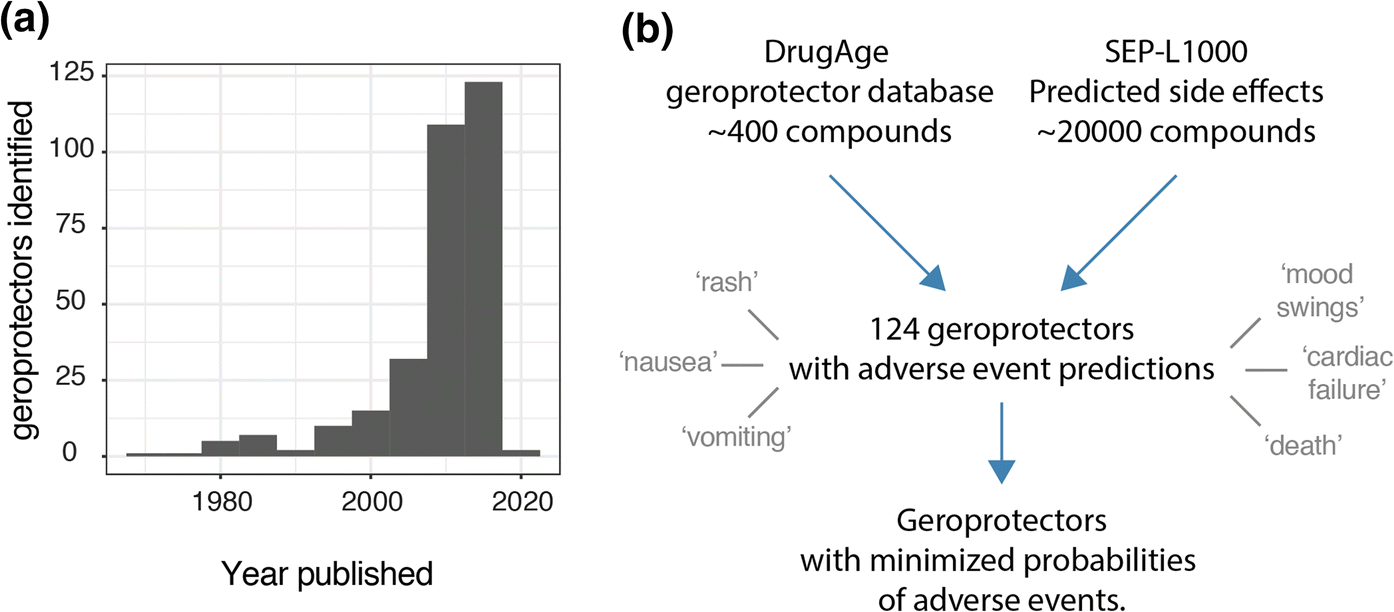
Fig. 1. Analysis approach. a Geroprotectors listed in DrugAge with histogram of publication dates. b Analysis approach whereby compounds from DrugAge were cross-referenced for their predicted side effects based on the SEP-L1000 predictions database. 124 compounds overlapped in this way and were assessed for their predicted side effects, which ranged from terms such as ‘rash’ to ‘death’.
However, the ultimate aim of these efforts is to identify compounds that promote healthy aging in humans. The translatability of geroprotectors from model organism studies to humans requires minimally that the compounds are safe for use, without causing serious side effects or health issues. This is not always the case for geroprotectors, even when assessed in model organisms. For example, the compound lamotrigine extends lifespan in flies, though the same study also found compromised health in treated animals (Avanesian et al. 2010). Following such examples, the translatability of geroprotectors from model organisms to humans has been an ongoing topic of discussion, with a need for criteria to prioritize compounds. Recent work in the field of aging research has consolidated a primary set of criteria to help prioritize geroprotectors for human use, which states that these compounds should have been demonstrated to (i) increase lifespan, (ii) ameliorate human aging biomarkers, (iii) have acceptable toxicity, (iv) cause minimal side effects at therapeutic dosage, and (v) improve health-related quality of life (Moskalev et al. 2016).
Minimizing side effects can be considered one of the highest priorities to answer the question ‘what known longevity interventions should we test in humans.’ This is especially true considering that geroprotectors may be distributed widely throughout the population to otherwise healthy individuals, and possibly for an extended period of time. Furthermore, prioritization of compounds for clinical testing is essential since evaluation of even a single compound, such as is the case with metformin in the TAME study, may require estimations of roughly 75 million USD in funding (De Grey 2019; Barzilai et al. 2016). While various curated efforts have selected candidate compounds to test in humans—such as with the selection of metformin (Barzilai et al. 2016; Moskalev et al. 2016)—a systematic account addressing potential side effects of known geroprotectors to date has been lacking. This is largely due to the fact that most geroprotectors identified thus far have been identified in model organisms and are compounds considered ‘for research use.’ These compounds therefore simply do not possess a large base of human users who register their side effects. For example, of the > 400 compounds cataloged in the DrugAge database of geroprotectors, only ~ 11% are from studies performed in vertebrates, while the vast majority are from invertebrate models, C. elegans specifically accounting for several hundreds of the compounds that have been identified. Similarly, over 80% of DrugAge’s compounds are not considered as approved for human use when cross-referencing with the listing of drugs in DrugBank (Wishart et al. 2018). Furthermore, though geroprotectors may be repurposed from already FDA approved drugs and therefore possess safety profiles, cross-comparison of registered side effects with other geroprotectors for systematic evaluation has not been thoroughly performed.
Recently, databases have been published that allow overcoming these main obstacles. In addition to the DrugAge database cataloging longevity compounds, an account of the probabilities of side effects of ~ 20,000 small molecules has also been published (Wang et al. 2016), based on the L1000 database of transcriptional signatures (Subramanian et al. 2017). The SEP-L1000 as it is called (for “side effect prediction based on L1000 data”), derives probabilities of side effects of compounds based on their transcriptional profiles in human cell culture. In conjunction with this, the SEP-L1000 was built, amongst other resources, also based on the ‘Side Effect Resource’ (SIDER) database of reported side effects in humans (Kuhn et al. 2016). We hypothesized that this SEP-L1000 database (Wang et al. 2016), paired with the well-established DrugAge database cataloging known geroprotectors (Barardo et al. 2017), could allow us to assess the risks of side effects of gerorpotectors in a systematic manner.
.../...
Results
Predicted side effects of geroprotectors
At the time that we accessed the DrugAge database, over 400 unique compounds were listed as extending lifespan in at least one model organism. Subsequently cross-referencing this against the SEP-L1000 database which contained ~ 20,000 compound’s predicted side effects, we found an overlap of 124 compounds to which we could assign 800 unique side effects (Fig. 1b). In order to explore the nature and prevalence of these side effects amongst geroprotectors, we ranked side effects according to how often they were predicted to occur (Fig. 2a). We found that nearly all (> 95%) geroprotectors in our list had a > 50% probability of causing side effects such as rashes (SIDER ID C0015230), nausea (SIDER ID C0027497), or vomiting (SIDER ID C0042963) (Fig. 2a, b). While these may be termed ‘mild’ side effects, they nonetheless would greatly decrease quality of life in otherwise healthy individuals. Furthermore, several geroprotectors, albeit few, also included much more severe side effects of mood swings (SIDER ID C0085633), cardiac failure (SIDER ID C0018801), or even death (SIDER ID C1306577) (Fig. 2b). Clearly, these are undesirable on the long term, with the side effect of ‘death’ even being something directly opposite to the goal of geroprotection. This serves to highlight the importance of selecting geroprotectors with few total side effects or low probabilities of causing such effects.
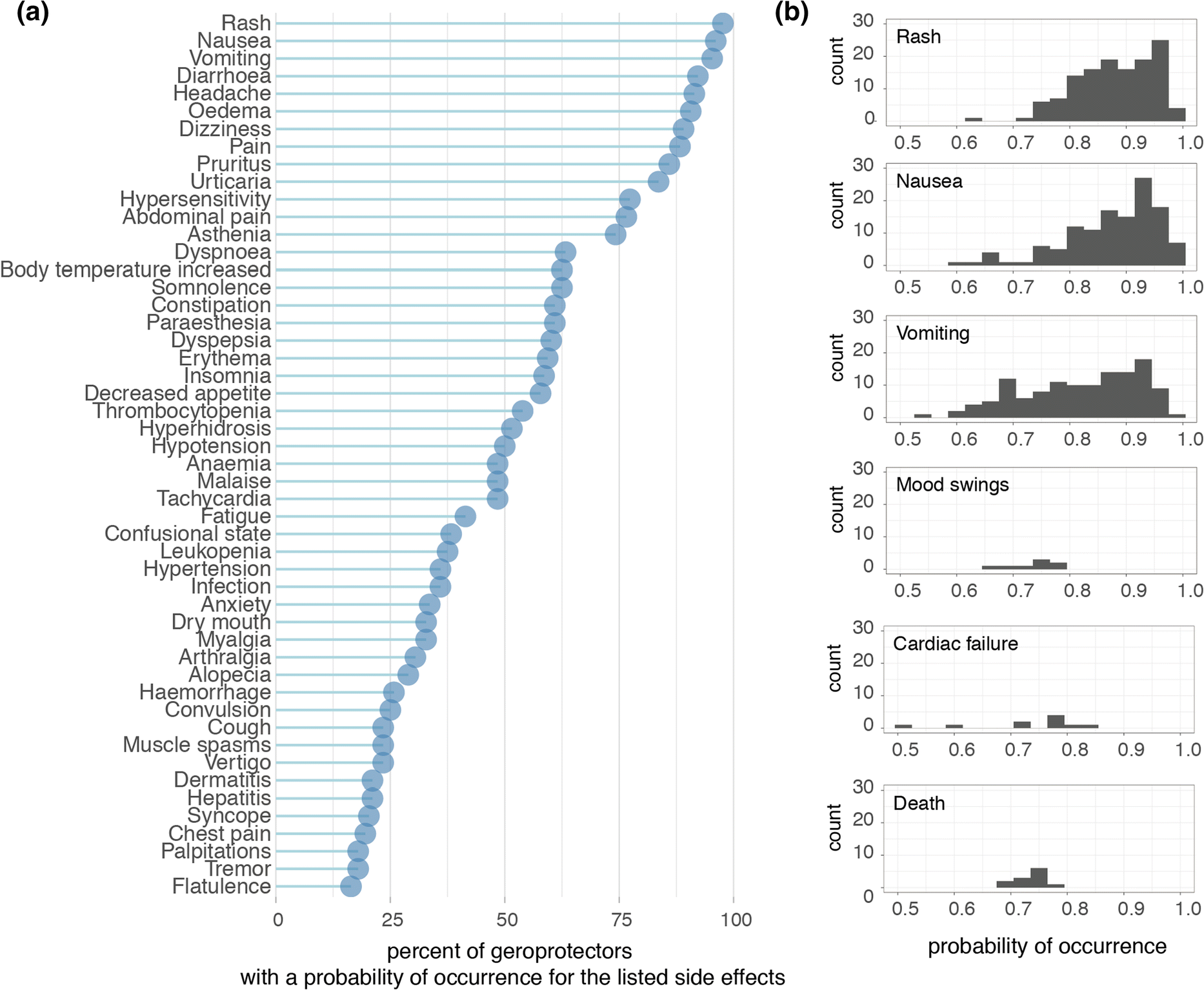
Fig. 2. Side effects of geroprotector compounds. a Top 50 side effects as ranked by percent of appearance within the 124 drugs. Graph depicts the percent of geroprotectors with a probability of occurrence for the listed side effects. The probability of rash, nausea, and vomiting occurs in nearly all geroprotectors, though each geroprotector may have a different probability for the occurrence of these side effects. The distributions of these probabilities for selected side effects are depicted in (b). b Examples of the distributions of probabilities for six selected side effects; rash, nausea, vomiting, mood swings, cardiac failure, and death.
Geroprotectors in general were not more prone to causing side effects compared to other compounds. When considering all 20,412 compounds in the SEP-L1000 database, similar probabilities of occurrence were observed for the most prominent side effects. Rash (associated with 20,198 compounds), nausea (associated with 20,166 compounds), and vomiting (associated with 19,937 compounds) were all predicted to occur for the vast majority of compounds (> 95%), while mood swings (associated with 149 compounds), cardiac failure (associated with 268 compounds), and death (associated with 198 compounds) were predicted for only a minority of the compounds (< 5%). Therefore, assessing side effects in geroprotectors serves rather to reveal and reinforce the fact that geroprotectors are not a drug class apart from others or free of side effects due to their longevity-inducing properties. Rather, careful and further consideration should be given to this class of compounds to ensure the lowest chance of side effects when used in humans.
In order to form a general overview of how dramatic an individual geroprotector’s risks of side effects were, we summarized for each geroprotector both the total number of side effects, and the highest probability that a side effect would be caused, according to the SEP-L1000 side effects database. Ranking compounds in these manners revealed those with both high and low risks for causing side effects (Fig. 3a, b). For example, lamotrigine, which was described to extend lifespan in flies though decrease health (Avanesian et al. 2010), had > 250 total predicted side effects listed, one of the highest in our ranking (Fig. 3a). Conversely, ascorbic acid (vitamin C), considered safe for humans even in high doses, was predicted to only cause three side effects (Fig. 3a). Likewise, lamotrigine had a nearly 100% maximum likelihood of causing a side effect, while spermidine, an autophagy inducer considered relatively safe for human use (Moskalev et al. 2016), carried only a 78% maximum likelihood of causing a side effect, the lowest in our analysis (Fig. 3b).
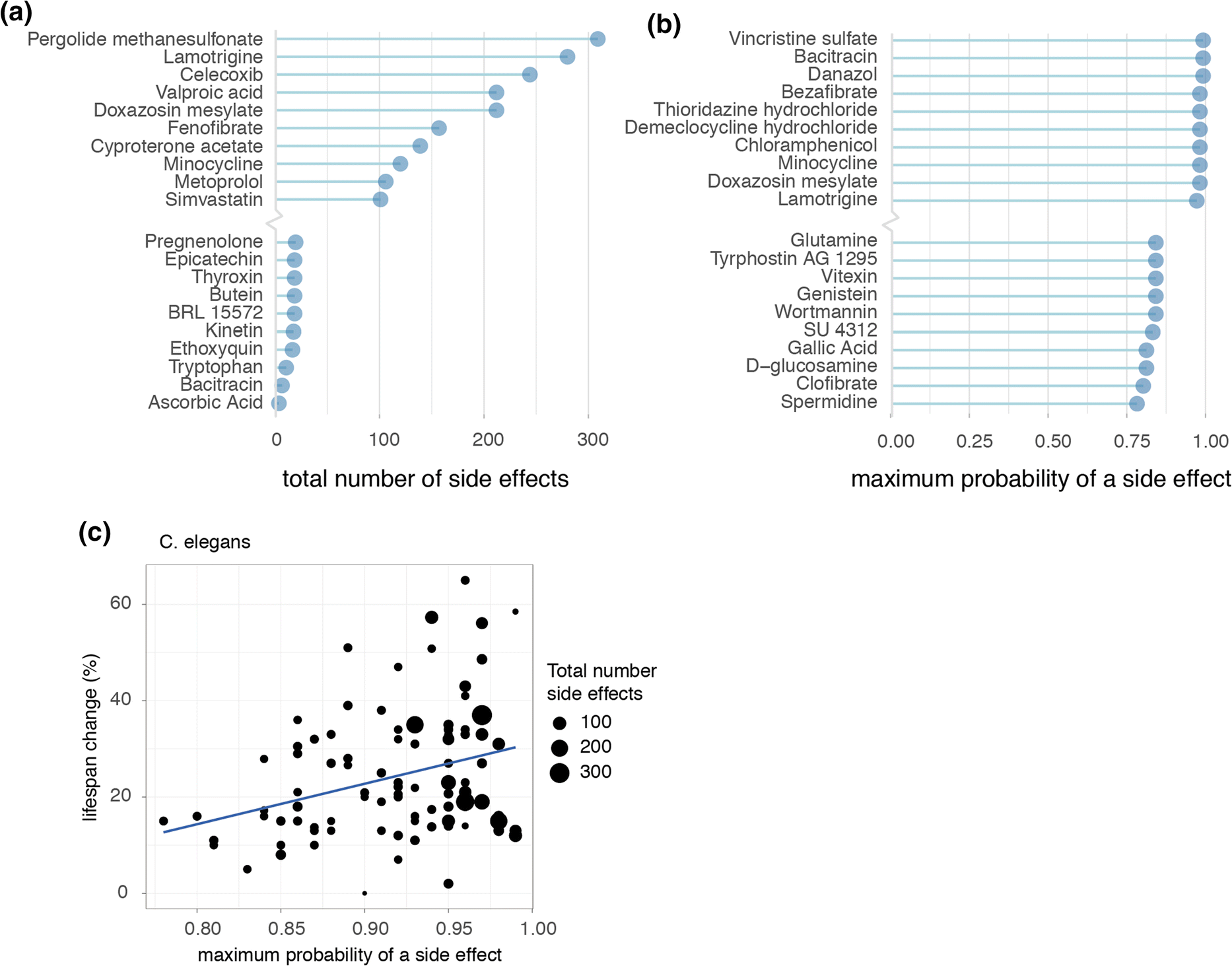
Fig. 3. Longevity effects of geroprotectors relate to maximum likelihoods of causing side effects. a Top 10 highest and lowest ranked geroprotectors in relation to the total number of side effects they are predicted to produce. b Top 10 highest and lowest ranked geroprotectors in relation to the maximum probability of a side effect. c Positive correlation observed in worms (C. elegans) between geroprotector’s lifespan extension and probability of causing a side effect (R = 0.32, p = 0.0018).
Assessing compounds in this way, we were able to inquire whether side effects in general possessed any correlation to the drug’s ability to increase lifespan. To address this, we turned to the C. elegans subset of DrugAge, since comparing geroprotector’s lifespan effects is more fairly performed within a single species, and C. elegans contained by far the largest repository of geroprotectors. Here, we plotted the maximum likelihood a compound had to cause a side effect relative to the percent it was able to increase lifespan. Remarkably, we found a significant positive correlation between a compound’s side effect risk and ability to increase lifespan (Fig. 3c) (R = 0.32, p < 0.01).












































