In Nature Communications, Ali Yücel and Vadim Gladyshev have published a review of the current state of the art in partial cellular reprogramming, detailing what this technology does and how it might be used safely.

Looking for Safety in Epigenetic Rejuvenation
#1
Posted 07 March 2024 - 05:06 PM
This paper begins by treading familiar ground on the subject, explaining its end goals and purpose. When successful, partial cellular reprogramming induces reprogramming-induced rejuvenation (RIR), a state in which a cell is transformed into an epigenetically younger cell of the same type and fulfilling the same function [1]. This process has had multiple crucial successes in experimental models, including human muscle cells [2] and skin cells [3] along with restoring vision [4] and extending lifespan [5] in mice.
Much of this work has been done in mice that have been genetically modified to express the necessary factors when doxycycline is administered. This has even been accomplished after birth via an adeno-associated virus (AAV) [5]. While there are four Yamanaka factors, OSKM, the fourth, c-Myc, is often omitted because it raises the risk of cancer. OSK administration significantly reduced the frailty of the treated mice.
As the authors note, applying these sorts of genetic modification techniques directly to human beings is currently infeasible with existing technologies. Partial reprogramming requires carefully determined generation of Yamanaka factors inside cells. To apply this in a clinical setting would require gene therapy that has specific and strong effects on individual tissues, and using the AAV system that works on mice is not yet practical for people [6]. Generating partially reprogrammed cells outside the body, similarly to how induced pluripotent stem cells (iPSCs) are generated, may be feasible for therapeutic purposes.
Small molecules raise possibilities and concerns
Administering small molecules to people in order to effect rejuvenation in the form of a drug has been the dream of aging researchers for some time. Previous work has spurred the creation of iPSCs through such chemical means [7]. The authors of this review describe these methods as less powerful than gene therapy and requiring multiple stages of administration. This implies a degree of safety and control that makes them more attractive for human research.
An experiment on mouse cells, which also included Vadim Gladyshev, had revealed that using a “7c” cocktail reduced multiple aspects of aging, including epigenetic clock measurements, age-related metabolic changes, and oxidative stress markers [8]. However, it also upregulates the senescence-associated p53 pathway, which is downregulated through normal reprogramming methods and may cause cells to become senescent earlier [9].
Tissue targeting may be crucial
Normally, constant expression of the Yamanaka factors in a living organism causes its cells to completely revert to a pluripotent state, in which they forget their roles, become cancerous, and cause the organism to die. For example, inducing OSKM for six days in the hearts of mice was found to be beneficial for them, while extending it for a dozen days proved lethal [10]. However, constantly inducing OSK in neural ganglion cells for a full 10-18 months improved vision without this side effect [4].
What is and isn’t improved
The authors note many of the aspects of aging that are improved or possibly improved with RIR, of which the most obvious, epigenetic alterations, is only one. Inflammation and proteostasis are also affected. Telomere attrition, however, occurs only in later reprogramming and is not affected by the partial variety [11]. Direct changes to cellular communication and genomic stability are not yet known.
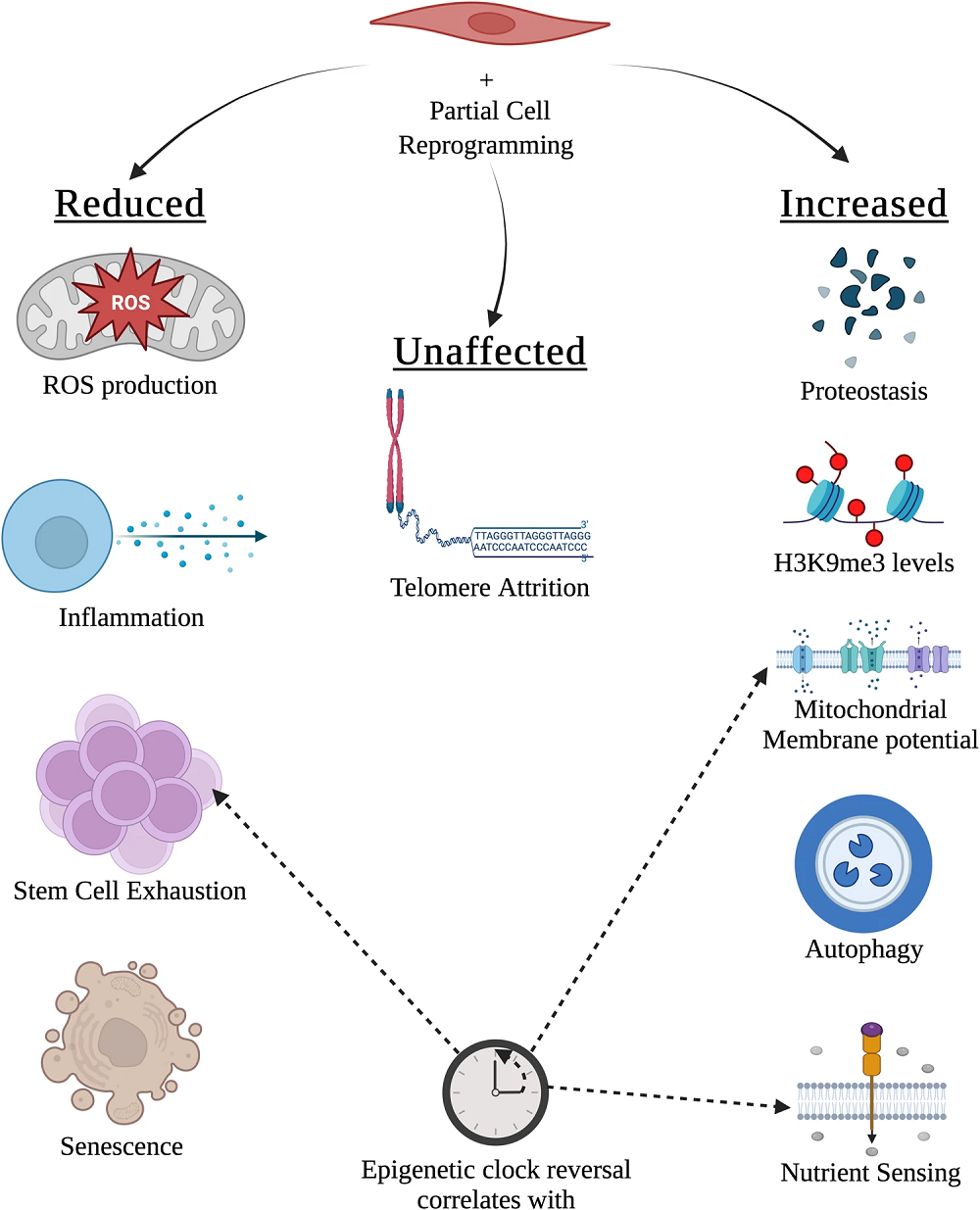
However, the authors point out that, while full reprogramming does not cells to mutate, creating colonies of iPSCs causes evolutionary pressure: cells with mutations that may not be beneficial for the whole organism may be more prevalent in iPSC colonies [12]. It remains to be seen if this is a concern for partial reprogramming.
The authors also mention a biochemical ‘pluripotency network’ and the fundamental differences between full and partial rejuvenation. Most critically, they hold that partial reprogramming is caused by factors that are downstream of full reprogramming. If it is possible to directly affect these factors instead of relying on the Yamanaka full-reprogramming factors, it might be possible to cause RIR without risking the dangerous side effects associated with complete reprogramming. However, this area of research remains unexplored.
Literature
[1] Ocampo, A., Reddy, P., Martinez-Redondo, P., Platero-Luengo, A., Hatanaka, F., Hishida, T., … & Belmonte, J. C. I. (2016). In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell, 167(7), 1719-1733.
[2] Sarkar, T. J., Quarta, M., Mukherjee, S., Colville, A., Paine, P., Doan, L., … & Sebastiano, V. (2020). Transient non-integrative expression of nuclear reprogramming factors promotes multifaceted amelioration of aging in human cells. Nature communications, 11(1), 1545.
[3] Gill, D., Parry, A., Santos, F., Okkenhaug, H., Todd, C. D., Hernando-Herraez, I., … & Reik, W. (2022). Multi-omic rejuvenation of human cells by maturation phase transient reprogramming. Elife, 11, e71624.
[4] Lu, Y., Brommer, B., Tian, X., Krishnan, A., Meer, M., Wang, C., … & Sinclair, D. A. (2020). Reprogramming to recover youthful epigenetic information and restore vision. Nature, 588(7836), 124-129.
[5] Macip, C. C., Hasan, R., Hoznek, V., Kim, J., Lu, Y. R., Metzger IV, L. E., … & Davidsohn, N. (2024). Gene Therapy-Mediated Partial Reprogramming Extends Lifespan and Reverses Age-Related Changes in Aged Mice. Cellular Reprogramming, 26(1), 24-32.
[6] Pupo, A., Fernández, A., Low, S. H., François, A., Suárez-Amarán, L., & Samulski, R. J. (2022). AAV vectors: The Rubik’s cube of human gene therapy. Molecular Therapy.
[7] Guan, J., Wang, G., Wang, J., Zhang, Z., Fu, Y., Cheng, L., … & Deng, H. (2022). Chemical reprogramming of human somatic cells to pluripotent stem cells. Nature, 605(7909), 325-331.
[8] Mitchell, W., Goeminne, L. J., Tyshkovskiy, A., Zhang, S., Chen, J. Y., Paulo, J. A., … & Gladyshev, V. N. (2023). Multi-omics characterization of partial chemical reprogramming reveals evidence of cell rejuvenation. bioRxiv, 2023-06.
[9] Tyner, S. D., Venkatachalam, S., Choi, J., Jones, S., Ghebranious, N., Igelmann, H., … & Donehower, L. A. (2002). p53 mutant mice that display early ageing-associated phenotypes. Nature, 415(6867), 45-53.
[10] Chen, Y., Lüttmann, F. F., Schoger, E., Schöler, H. R., Zelarayán, L. C., Kim, K. P., … & Braun, T. (2021). Reversible reprogramming of cardiomyocytes to a fetal state drives heart regeneration in mice. Science, 373(6562), 1537-1540.
[11] Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. cell, 126(4), 663-676.
[12] Kosanke, M., Osetek, K., Haase, A., Wiehlmann, L., Davenport, C., Schwarzer, A., … & Martin, U. (2021). Reprogramming enriches for somatic cell clones with small-scale mutations in cancer-associated genes. Molecular Therapy, 29(8), 2535-2553.
View the article at lifespan.io