Instead of using cellular reprogramming to directly treat age-related diseases, a perspective published in Nature Communications focuses on the opposite: using reprogrammed cells to form aged tissues and organoids on which to conduct experiments.
Why not just use donors?
Obviously, there is no shortage of age-related tissues in the world, and tissue donors can and do donate samples on which to conduct studies on potential treatments for age-related diseases [1]. However, there are limitations: these donors need to be extensively studied, and large samples from such organs as the brain, heart, and blood vessels can’t be obtained from living donors. Additionally, cells taken from these donors lack intercellular communication between other tissues when removed, the physical topography on which they are grown is different from in living tissues, and it is difficult to grow a large number of somatic cells due to the telomere-related Hayflick limit on their reproduction.
Of course, cells can be encouraged to freely divide by using the Yamanaka factors to induce pluripotency, returning them to stem cells. However, this is somewhat counterproductive when the subject being studied is aging: the cells are epigenetically rejuvenated in the process, which is exactly what these researchers don’t want. This review, then, focuses on ways in which this fact can be circumvented to create models of aging.
Inducing aging in iPSCs
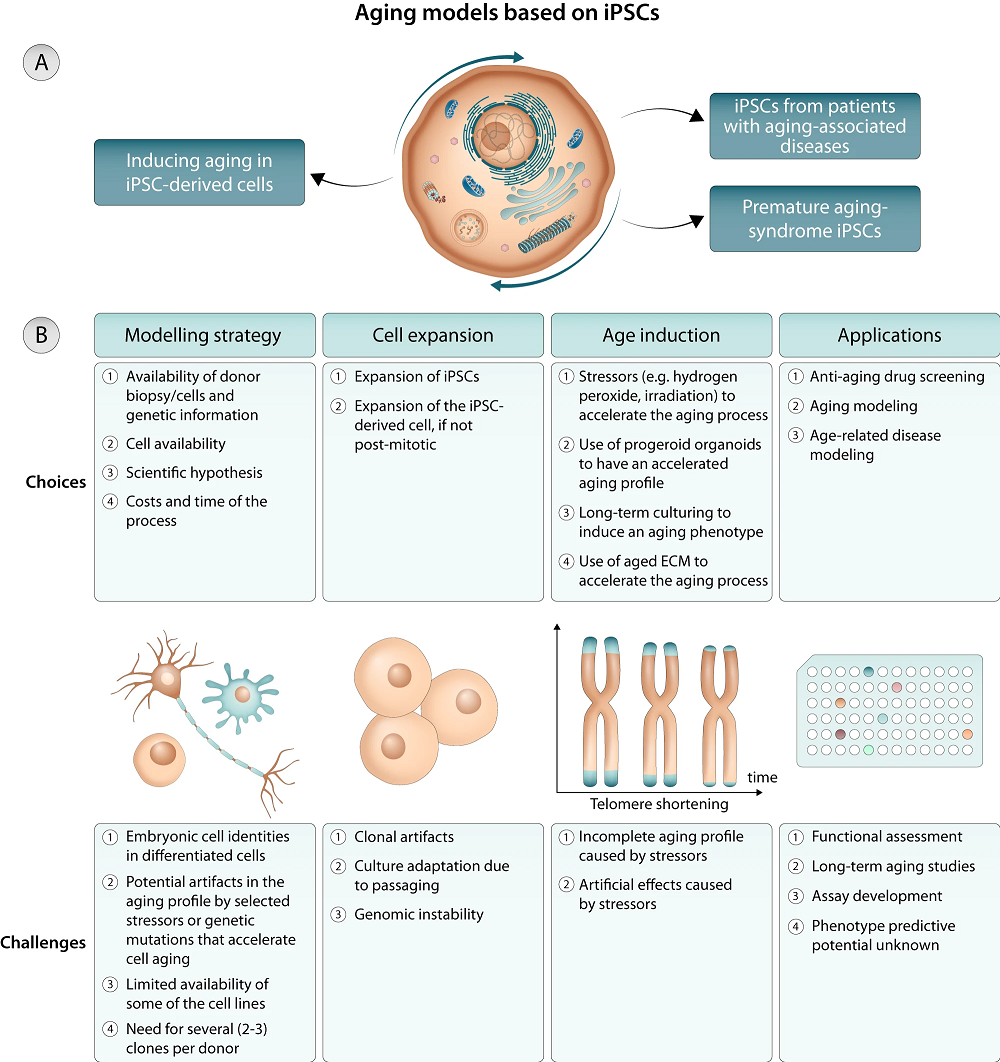
Fortunately for this particular line of research, and very unfortunately for humans as a whole, there are many ways to induce aging in cells. Simply culturing them for a long time is an option [2], although it it is not always feasible. Exposing heart muscle cells to an aged extracellular matrix (ECM) ages them within a few months [3]. Inducing telomere shortening through telomerase manipulation is another option [4].
It is also possible to use cells derived from people with age-related genetic problems, such as progeria [5] and a mutation related to accelerated heart disease [6]. These reviewers note that even with those cells, experiments often artificially expose them to additional stress, such as oxidative stress, to induce aging more rapidly [7]. While informative, these experiments do not fully reflect normal aging.
Direct transdifferentiation
Because some cell types are far easier to obtain than others, cells are directly transdifferentiated from one type to another through microRNAs and cellular signals. This process does not induce pluripotency, instead retaining the original aged epigenetics [8]. Normally, skin fibroblasts are used to convert into other cell types, and these cells originate from multiple sources from one individual, reflecting a natural genetic mosaicism that is not found in iPSC-derived clonal cultures.
However, this aged source poses its own problem for researchers. There can be no ‘line’ of these cells, as there are for iPSC-derived cultures and cancer cells. Regular fibroblast donations are required for experiments with transdifferentiated cells to continue. It is also not feasible to genetically engineer these cells in the same way that iPSCs can be modified.
2D or 3D
The authors also talk about two-dimensional versus three-dimensional cell cultures. Two-dimensional cell cultures are easier to create and to scale up, and they can still give valuable insights such as in the study on cells exposed to an aged ECM [3]. However, they are still communicating primarily with the substrate under them rather than other cells, as they would be in an actual tissue.
This poses a problem when trying to model age-related issues such as the SASP, the inflammatory chemicals that senescent cells secrete and that drive more cells senescent. In a 3D organoid model, the SASP would be transmitted in a way that could facilitate research into dealing with it.
Additionally, physical effects such as biomechanical forces can’t be studied in a 2D model. However, iPSCs have been artificially aged and placed onto a 3D chip in order to study the effects of physical forces on these cells [9]. Further studies involve organoids, which are constructs made from multiple types of cells, just as they are in living organisms, and research has been done into using them to study aging [10], including brain aging [11].
While these organoids are not human beings, they are also not mouse models. Both approaches have their own limitations and concerns, and both will be needed to adequately test prospective therapies before they reach clinical trials.
Literature
[1] Brunet, A. (2020). Old and new models for the study of human ageing. Nature Reviews Molecular Cell Biology, 21(9), 491-493.
[2] Odawara, A., Katoh, H., Matsuda, N., & Suzuki, I. (2016). Physiological maturation and drug responses of human induced pluripotent stem cell-derived cortical neuronal networks in long-term culture. Scientific reports, 6(1), 26181.
[3[ Ozcebe, S. G., Bahcecioglu, G., Yue, X. S., & Zorlutuna, P. (2021). Effect of cellular and ECM aging on human iPSC-derived cardiomyocyte performance, maturity and senescence. Biomaterials, 268, 120554.
[4] Vera, E., Bosco, N., & Studer, L. (2016). Generating late-onset human iPSC-based disease models by inducing neuronal age-related phenotypes through telomerase manipulation. Cell reports, 17(4), 1184-1192.
[5] Eriksson, M., Brown, W. T., Gordon, L. B., Glynn, M. W., Singer, J., Scott, L., … & Collins, F. S. (2003). Recurrent de novo point mutations in lamin A cause Hutchinson–Gilford progeria syndrome. Nature, 423(6937), 293-298.
[6] Chang, A. C., Chang, A. C., Kirillova, A., Sasagawa, K., Su, W., Weber, G., … & Blau, H. M. (2018). Telomere shortening is a hallmark of genetic cardiomyopathies. Proceedings of the National Academy of Sciences, 115(37), 9276-9281.
[7] Reinhardt, P., Schmid, B., Burbulla, L. F., Schöndorf, D. C., Wagner, L., Glatza, M., … & Sterneckert, J. (2013). Genetic correction of a LRRK2 mutation in human iPSCs links parkinsonian neurodegeneration to ERK-dependent changes in gene expression. Cell stem cell, 12(3), 354-367.
[8] Mertens, J., Paquola, A. C., Ku, M., Hatch, E., Böhnke, L., Ladjevardi, S., … & Gage, F. H. (2015). Directly reprogrammed human neurons retain aging-associated transcriptomic signatures and reveal age-related nucleocytoplasmic defects. Cell stem cell, 17(6), 705-718.
[9] Pitrez, P. R., Estronca, L., Monteiro, L. M., Colell, G., Vazão, H., Santinha, D., … & Ferreira, L. (2020). Vulnerability of progeroid smooth muscle cells to biomechanical forces is mediated by MMP13. Nature communications, 11(1), 4110.
[10] Hu, J. L., Todhunter, M. E., LaBarge, M. A., & Gartner, Z. J. (2018). Opportunities for organoids as new models of aging. Journal of Cell Biology, 217(1), 39-50.
[11] Shakhbazau, A., Danilkovich, N., Seviaryn, I., Ermilova, T., & Kosmacheva, S. (2019). Effects of minocycline and rapamycin in gamma-irradiated human embryonic stem cells-derived cerebral organoids. Molecular biology reports, 46(1), 1343-1348.
View the article at lifespan.io









































