A team of researchers has waded into a controversial and contradictory area of study, publishing information on the link between obesity and an inflammatory molecule that increases with aging.

An Inflammatory Molecule May Also Encourage Obesity
#1
Posted 15 April 2024 - 04:05 PM
The authors point out previous research that singled out the interleukin IL-6, a key immune signaling molecule, as a major contributor to inflammaging [1]. However, its role in biology is complicated, and much of the relevant research is contradictory: it has been reported to have both pro- and anti-inflammatory functions, depending on context [2], and it plays multiple roles in metabolism [3].
Adding to this confusion, some research has found that it enhances insulin secretion in muscle tisssue [4], while other research has found that it increases insulin resistance in the liver [5]. Most importantly for this study, previous work has found that it stimulates fat burning [6]. These researchers, however, have come to the opposite conclusion: that IL-6 inhibits fat burning and promotes obesity instead.
Beginning with humans, moving on to mice
This work began by studying 77 hospitalized patients who had type 2 diabetes and were at least 65 years old. The third of this group that had the most IL-6 also had significantly more fat on their organs than the other two thirds. This was found to be true even when adjusting for many known confounders, such as age, disease length, other metabolic and diabetes biomarkers, and vascular issues.
However, those human results are only a correlation. In an attempt to prove causation, they moved to an animal model. Older wild-type mice naturally express more IL-6 than younger wild-type mice, but compared to a genetically modified model that does not express IL-6 at all, they found no significant differences in lifespan, liver function, insulin use, or glucose.
With aging, older wild-type mice also naturally gain more weight, but this weight gain was significantly blunted in mice that did not express IL-6. These modified mice also had somewhat less skin fat and far less fat around their organs, along with less cholesterol and fewer triglycerides. Wild-type mice expend less energy with age; this change, too, was significantly blunted in the IL-6-deficient mice. Gene expression analysis found that a deficiency in IL-6 meant that pathways related to fat burning were upregulated compared to the wild-type group.
A closer look discovered that, in older wild-type mice, fat cells near organs can become significantly larger than normal. This was found to be the reason why IL-6 deficiency resulted in so much less organ fat: the IL-6-deficient mice accumulated far fewer of these large fat cells.
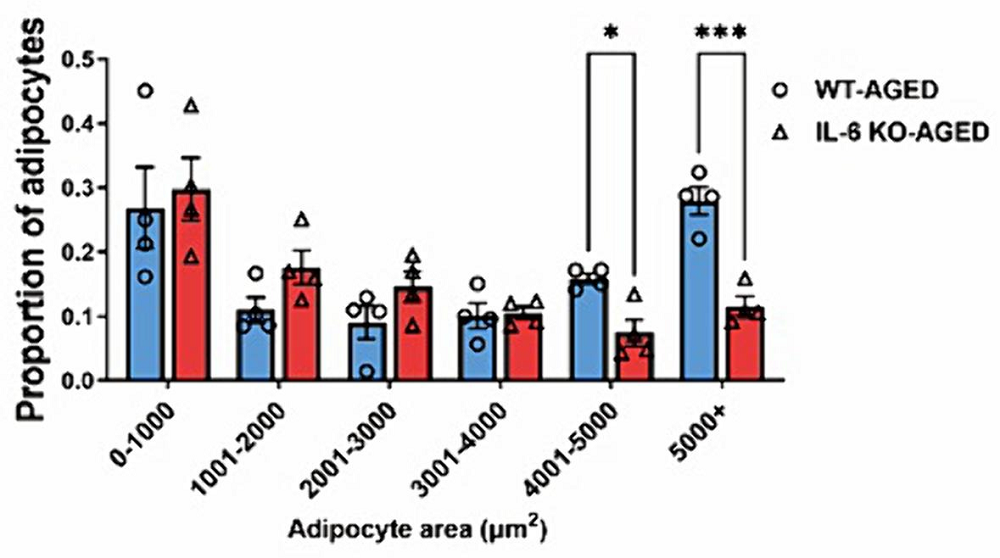
Age-related effects in mice, but questionable for people
Most importantly, all of these differences were only found between the aged mice in each group. There were no significant differences between young mice in either group. However, as IL-6 is a core signaling molecule with well-documented effects, attempting to completely suppress IL-6 in people would be likely to do more harm than good.
These researchers suggest that the effects of IL-6 are largely cell-dependent and that these particular detrimental effects are specific to fat tissue. Biology is not fully mapped, but in this case, it is likely that a far more complete map of the true cause-and-effect biochemical relationship between interleukins and fat tissue would need to be created before this line of research could result in drug development.
Literature
[1] Gabay, C. (2006). Interleukin-6 and chronic inflammation. Arthritis research & therapy, 8, 1-6.
[2] Scheller, J., Chalaris, A., Schmidt-Arras, D., & Rose-John, S. (2011). The pro-and anti-inflammatory properties of the cytokine interleukin-6. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1813(5), 878-888.
[3] Giraldez, M. D., Carneros, D., Garbers, C., Rose-John, S., & Bustos, M. (2021). New insights into IL-6 family cytokines in metabolism, hepatology and gastroenterology. Nature reviews Gastroenterology & hepatology, 18(11), 787-803.
[4] Ellingsgaard, H., Hauselmann, I., Schuler, B., Habib, A. M., Baggio, L. L., Meier, D. T., … & Donath, M. Y. (2011). Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nature medicine, 17(11), 1481-1489.
[5] Sabio, G., Das, M., Mora, A., Zhang, Z., Jun, J. Y., Ko, H. J., … & Davis, R. J. (2008). A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science, 322(5907), 1539-1543.
[6] Van Hall, G., Steensberg, A., Sacchetti, M., Fischer, C., Keller, C., Schjerling, P., … & Pedersen, B. K. (2003). Interleukin-6 stimulates lipolysis and fat oxidation in humans. The Journal of Clinical Endocrinology & Metabolism, 88(7), 3005-3010.
View the article at lifespan.io