The Hallmarks of Aging team has returned to Cell, publishing a detailed review discussing how future methods of dealing with aging might be highly personalized.
Two more hallmarks?
In the original and very heavily cited 2013 paper [1], Dr. López-Otín and colleagues outlined nine hallmarks of aging. In their 2023 update [2], three more were added, reflecting the enhanced state of knowledge on the subject. Here, they add two more, bringing the total to 14: changes to the extracellular matrix (ECM), which are well-documented to occur with aging [3], along with psychosocial isolation, which is both a cause and consequence of age-related mental and physical enfeeblement [4].
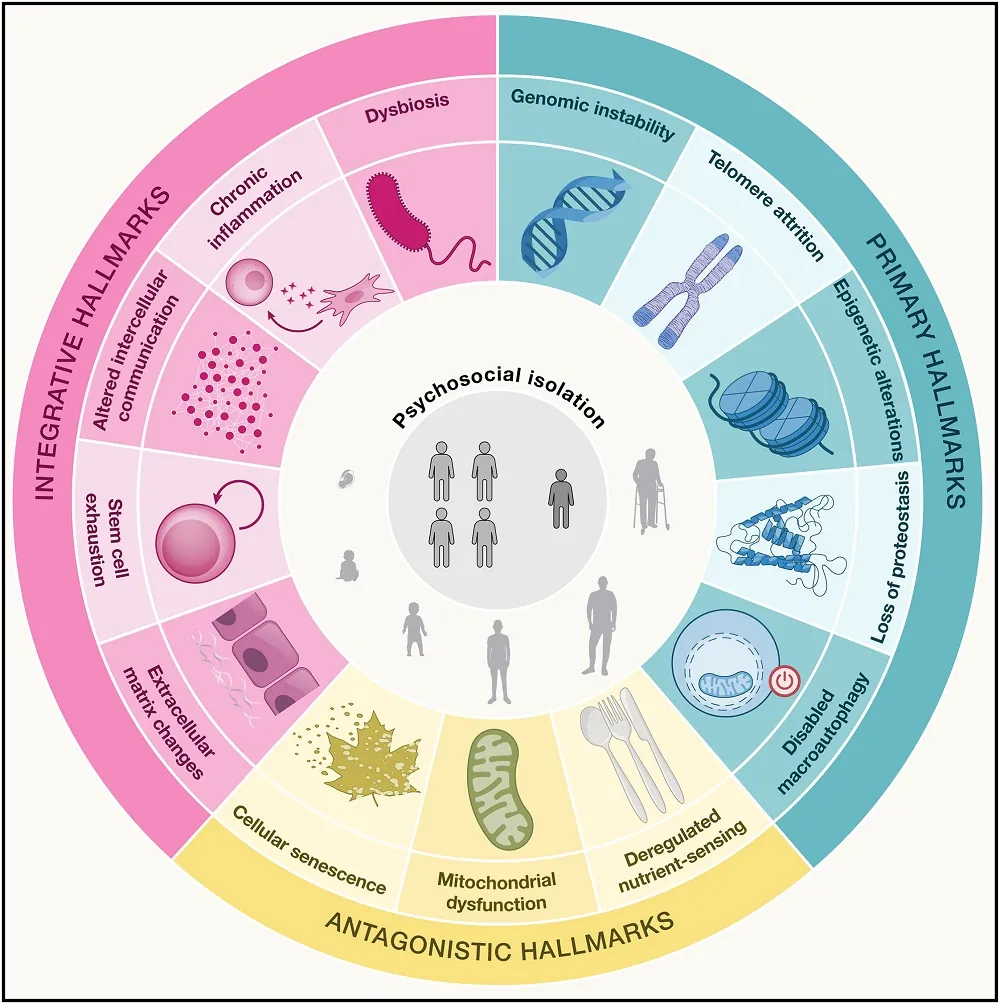
However, this paper notes the fundamental limitations of the hallmarks, one of the largest being that they are so heavily intertwined. They give the example of restoring autophagy, which assists genomic stability, restores mitochondrial function, improves intestinal barrier function, and reduces inflammation. The downstream effects of dealing with senescent cells are similar. Therefore, they refer to the hallmarks as “entry points” that researchers can use to attack aging from multiple angles.
The reviewers also lament the difficulty of using individual hallmarks as biomarkers. Beccause of senescent cells’ heterogeneity, detailed -omics techniques are the only useful way of measuring them, and the same is true for inflammation and intestinal dysbiosis. There is no established way to measure whole-organism autophagy at all. This lack of standardization makes it difficult to establish continuity between experiments.
Gerosuppressors versus gerogenes
Because of the difficulty of using hallmarks as diagnostic tools, the researchers offer an entirely different method of evaluating aging: one focused on genes [5]. This method allows for more precise evaluation and is not dependent on molecular pathways.
The authors point out that these genes are not evolved to promote or fight aging; rather, the functions they perform have downstream consequences that lead to longevity or its inverse. Some of these genes are well-known, such as the APOE variants associated with Alzheimer’s or its prevention [6] and a mutation of the lamin A gene leading to progeria [7], but many genetic mutations can accelerate aging in some way. To be defined as a gerogene or a gerosuppressor, a gene must both be associated with human aging and have effects when intervened upon in animal studies. Ideally, confirmation will be established once these treatments have been tested in human clinical trials.
The reviewers also express the hope that the massive amount of data in biomarkers can be functionally evaluated by an AI system [8]. These encompass epigenetic clocks along with a great many -omics, including evaluations of cell ratios, microbiomes, plasma proteins, and metabolism. It is unknown whether or not an algorithm trained on these masses of data will be better than more foundational biology in identifying and evaluating molecular targets; however, they may be more immediately useful in diagnosing the effects of existing drugs and the various drugs currently in clinical trials.
Additionally, aging need not always affect the whole body at the same time. The reviewers describe the phenomenon of localized aging, in which individual organs age faster than the rest of the body, increasing mortality risk in otherwise generally healthy people [9]. They also discuss ageotypes, molecular signatures that describe how someone is aging [10], although these ageotypes are not currently well-defined. Future, AI-driven analysis may better describe this phenomenon.
Bringing precision geromedicine to the clinic
The reviewers offer a three-pronged strategy for evaluating and treating aging on the individual level. The first is to use a systems biology approach, using a full suite of -omics technologies and clinical evaluations in order to suggest particular treatments. Second, premature aging (“precocious derangements”) should be discovered and treated in advance of clinical pathology. Third, biomarker analysis should be used to detect specific problems, such as cancer and blood vessel blockages, well in advance.
Obviously, much of this evaluation and prevention is part of ordinary clincal practice today; however, such diagnostics and treatment are often performed by specialists in individual areas rather than being based on systemic evaluations. The reviewers also strongly differentiate their systemic approach from ‘longevity clinics’, which frequently offer untested therapies. Instead, the reviewers argue, a ‘longevity clinic’ should be part of a hospital and rely solely on treatments and diagnostics that are supported by evidence and will not be harmful to their patients.
The reviewers also offer a method by which this approach might be approved by the FDA. They propose that, alongside a control group receiving the current standard of medical care, healthy people should be given access to care that uses individualized multi-omics and interventions, some of which are non-medical in nature (such as diet and exercise). The evaluation criteria would be quantifiable differences in aging biomarkers. Naturally, such a trial would be extremely expensive, which the reviewers acknowledge, suggesting that it should be conducted with resources in multiple countries.
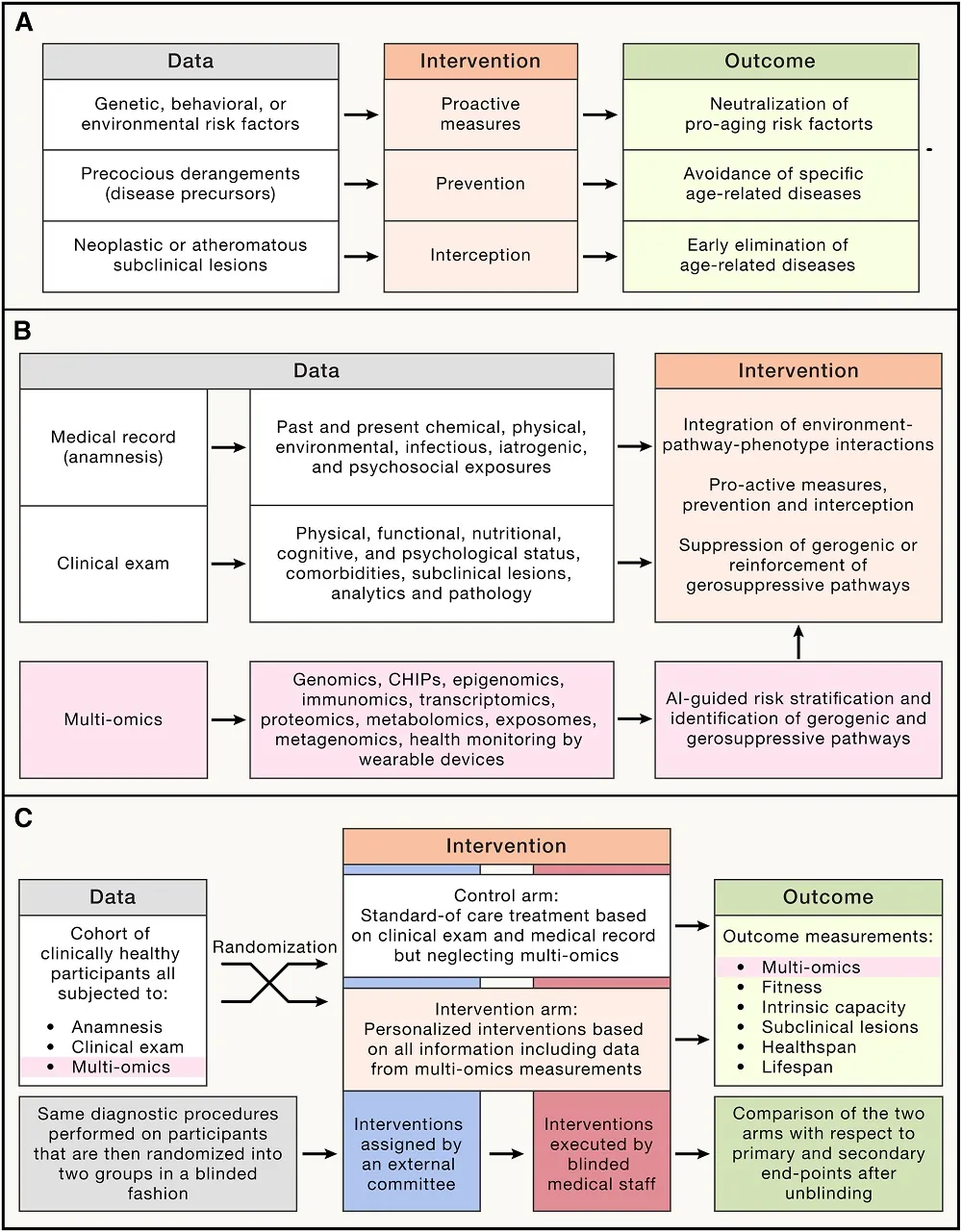
Ultimately, this is a blueprint and a suggestion for updating the existing medical framework with technological advancements that may extend patients’ healthy lifespan. If -omics approaches can be brought to the clinic and widely adopted by doctors, it may be easier and more feasible for functional anti-aging interventions to reach the public.
Literature
[1] López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2013). The hallmarks of aging. Cell, 153(6), 1194-1217.
[2] López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2023). Hallmarks of aging: An expanding universe. Cell, 186(2), 243-278.
[3] Mavrogonatou, E., Papadopoulou, A., Pratsinis, H., & Kletsas, D. (2023). Senescence-associated alterations in the extracellular matrix: deciphering their role in the regulation of cellular function. American Journal of Physiology-Cell Physiology, 325(3), C633-C647.
[4] López-Otín, C., & Kroemer, G. (2024). The missing hallmark of health: psychosocial adaptation. Cell stress, 8, 21.
[5] López-Otín, C., Maier, A. B., & Kroemer, G. (2024). Gerogenes and gerosuppression: the pillars of precision geromedicine. Cell Research, 34(7), 463-466.
[6] Neel, J. V. (1962). Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”?. American journal of human genetics, 14(4), 353.
[7] Gordon, L. B., Rothman, F. G., López-Otín, C., & Misteli, T. (2014). Progeria: a paradigm for translational medicine. Cell, 156(3), 400-407.
[8] Biomarkers of Aging Consortium, Herzog, C. M., Goeminne, L. J., Poganik, J. R., Barzilai, N., Belsky, D. W., … & Gladyshev, V. N. (2024). Challenges and recommendations for the translation of biomarkers of aging. Nature Aging, 4(10), 1372-1383.
[9] Oh, H. S. H., Rutledge, J., Nachun, D., Pálovics, R., Abiose, O., Moran-Losada, P., … & Wyss-Coray, T. (2023). Organ aging signatures in the plasma proteome track health and disease. Nature, 624(7990), 164-172.
[10] Ahadi, S., Zhou, W., Schüssler-Fiorenza Rose, S. M., Sailani, M. R., Contrepois, K., Avina, M., … & Snyder, M. (2020). Personal aging markers and ageotypes revealed by deep longitudinal profiling. Nature medicine, 26(1), 83-90.
View the article at lifespan.io









































