Scientists have created engineered nanostructures that bind monomers and oligomers of harmful amyloid beta (Aβ) protein, preventing them from entering neurons and drastically increasing the cells’ survival in vitro [1].
Don’t let them into cells!
Misfolded proteins are thought to be behind diseases like Alzheimer’s and amyotrophic lateral sclerosis (ALS). The most recognizable hallmark of Alzheimer’s is the aggregation of amyloid plaques between brain cells. However, removing those plaques has only very limited impact on the disease.
Recently, evidence has been growing that soluble Aβ early-stage fibrils and oligomers, which can enter cells, are more damaging than plaques and more tightly linked to cognitive decline [2]. Moreover, plaques might act as a sink, pulling harmful oligomers out of circulation [3]. Scientists have tried targeting these harmful proteins using antibodies, but clearly, new, better chemical tools are needed.
A new study from Northwestern University, published in the Journal of the American Chemical Society (ACS), explores one such tool: engineered peptide amphiphiles (TPAs). These are molecules that can self-assemble into long nanofibers through a process called supramolecular polymerization. Some TPAs, such as semaglutide, are already used in therapies.
Neuronal death averted
The researchers ingeniously combined several building blocks to create custom fibers designed to bind to Aβ. Those included short chains of amino acids (peptides) and a natural sugar called trehalose.
“The advantage of peptide-based drugs is that they degrade into nutrients,” said Dr. Samuel I. Stupp, the study’s senior author. “The molecules in this novel therapeutic concept break down into harmless lipids, amino acids, and sugars. That means there are fewer adverse side effects.”
The researchers thought that trehalose might stabilize misfolded proteins, since it is known as a protein chaperone that can protect proteins from misfolding, denaturation, and aggregation [4].
“Trehalose is naturally occurring in plants, fungi, and insects,” said Zijun Gao, a Ph.D. candidate in Stupp’s laboratory and the paper’s first author. “It protects them from changing temperatures, especially dehydration and freezing. Others have discovered that trehalose can protect many biological macromolecules, including proteins. So, we wanted to see if we could use it to stabilize misfolded proteins.”
To the scientists’ surprise, trehalose actually destabilized the nanofibers, making them “metastable” and more prone to binding to surrounding molecules, specifically Aβ42 peptides, a particularly harmful subspecies. Instead of just blocking the process, the nanofibers physically trapped the Aβ42 peptides by incorporating them into their structure.
“Unstable assemblies of molecules are very reactive,” Stupp explained. “They want to interact with and bond to other molecules. If the nanofibers were stable, they would happily ignore everything around them.”
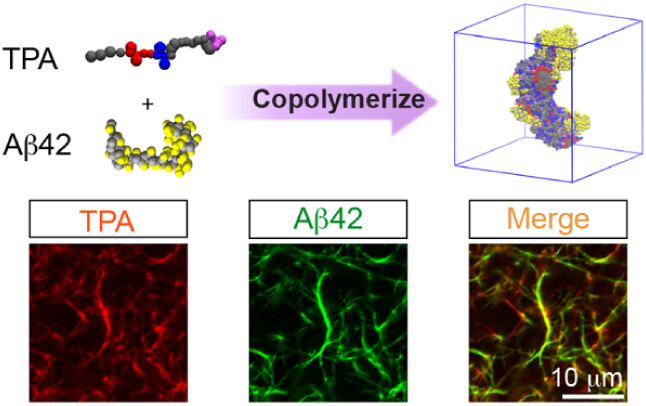
The researchers co-cultured Aβ42 with human neurons derived from induced pluripotent stem cells (iPSCs). Using fluorescence microscopy, they observed that in the presence of TPA, Aβ42 did not accumulate in neuronal lysosomes, directly correlating with dramatically improved survival of neurons and demonstrating that the entrapment blocked Aβ42 uptake into them. While co-culturing with Aβ42 but not TPA caused more than 60% of cells to die, the presence of TPA reduced cell death to that of healthy controls.
“Our study highlights the exciting potential of molecularly engineered nanomaterials to address the root causes of neurodegenerative diseases,” said Stupp. “By trapping the misfolded proteins, our treatment inhibits the formation of those fibers at an early stage. Early-stage, short amyloid fibers, which penetrate neurons, are believed to be the most toxic structures. With further work, we think this could significantly delay progression of the disease.”
Lots of further questions
While these results are encouraging, significant questions remain. First, is it possible to deliver TPA structures, which might be too big to cross the blood-brain barrier, into the central nervous system? The researchers suggest that one of Alzheimer’s symptoms, increased blood-brain barrier permeability, might help. Alternatively, delivery via the intranasal route, which forgoes the BBB entirely, might be used.
Whether clearance of TPA-Aβ42 conjugates from the brain would be required, and if so, how this can be achieved, is another potential issue. Finally, the study did not assess effects on the extracellular environment beyond blocking Aβ42 internalization by neurons. In particular, there was no measurement of inflammatory signaling, extracellular toxicity markers, membrane disruption, or interactions with non-neuronal glial cells.
The researchers note that their invention might revolutionize Alzheimer’s treatment, especially at an early stage before large amounts of Aβ42 have accumulated inside neurons. This highlights the need for early-stage Alzheimer’s screening, a hot research topic in which some recent advances have been made [5].
Literature
[1] Gao, Z., Qiu, R., Dave, D. R., Chandravanshi, P., Soares, G. P., Smith, C. S., … & Stupp, S. I. (2025). Supramolecular Copolymerization of Glycopeptide Amphiphiles and Amyloid Peptides Improves Neuron Survival. Journal of the American Chemical Society.
[2] Hermann, D., Both, M., Ebert, U., Gross, G., Schoemaker, H., Draguhn, A., … & Nimmrich, V. (2009). Synaptic transmission is impaired prior to plaque formation in amyloid precursor protein–overexpressing mice without altering behaviorally-correlated sharp wave–ripple complexes. Neuroscience, 162(4), 1081-1090.
[3] Fagan, A. M., Mintun, M. A., Mach, R. H., Lee, S. Y., Dence, C. S., Shah, A. R., … & Holtzman, D. M. (2006). Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Annals of neurology, 59(3), 512-519.
[4] Vinciguerra, D., Gelb, M. B., & Maynard, H. D. (2022). Synthesis and application of trehalose materials. Jacs Au, 2(7), 1561-1587.
[5] Palmqvist, S., Warmenhoven, N., Anastasi, F., Pilotto, A., Janelidze, S., Tideman, P., … & Hansson, O. (2025). Plasma phospho-tau217 for Alzheimer’s disease diagnosis in primary and secondary care using a fully automated platform. Nature Medicine, 1-8.








































