Scientists have analyzed the differences between mammalian species that can regrow ear tissue after injury and those that cannot. Their findings can pave the way for novel regenerative therapies [1].
The lost art of regeneration
Many animal species have amazing regenerative abilities. On the one side of the spectrum sit planarian worms: slice them up, and every slice will grow into a fully developed animal. Even more complex animals, such as amphibians, can fully regenerate limbs. Mammals, however, have largely lost this ability. If only we could “teach” human tissues how to regenerate, this would open completely new horizons for anti-aging therapies.
Interestingly, some mammals have retained certain regenerative potential. For instance, rabbits can fully regenerate damaged outer ear (“ear pinna”) tissue, while mice and rats cannot. In a new study published in Science, a team of Chinese researchers set out to discover what sets those species apart when it comes to regeneration.
One master regulator
First, they punched holes in the ears of three regenerating species (rabbits, goats, and African spiny mice), and two non-regenerating ones (laboratory mice and rats). As expected, in the first group, the injured ears fully regenerate, including the cartilage. In mice and rats, however, only the wound boundaries heal, leaving the hole.
However, the team made a crucial observation: the initial stages were surprisingly similar. Both species formed a blastema: a mass of formerly specialized cells, such as skin and muscle cells, that dedifferentiated into a more stem-like state to facilitate regeneration and initially showed robust cell proliferation. The difference was that in non-regenerative species, the process was weaker and soon petered out.
This showed that the failure in mice and rats wasn’t an inability to start the regenerative process but an inability to sustain it. Next, the researchers focused on finding the molecular causes for those differences in regenerative capacity.
Using state-of-the-art techniques, including single-cell RNA sequencing (scRNA-seq) and spatial transcriptomics, on the healing ear tissue, the team ran a deep comparison between rabbits and mice. The most significant differences in gene expression were found in wound-induced fibroblasts (WIFs), a subpopulation of fibroblasts that appeared only after tissue damage in both species.
The researchers ultimately zeroed in on nine regeneration-associated genes (RAGs) that were differentially expressed in the WIFs of regenerative and non-regenerative species and ran a series of experiments, overexpressing some of the top candidates in mice using viral delivery. One gene, Aldh1a2, was sufficient to fully restore ear pinna regeneration. The team then confirmed through RNA analysis that Aldh1a2 was strongly activated following injury in rabbits, goats, and African spiny mice but barely detectable in mice and rats.
The gene produces aldehyde dehydrogenase 1 family member A2, a rate-limiting enzyme in the synthesis of retinoic acid (RA), a known regeneration factor. Retinoic acid’s precursor is vitamin A (retinol).
Systemically treating mice with retinoic acid boosted ear regeneration. Retinol, on the other hand, did not have that effect, because in retinoic acid synthesis, it lies upstream of ALDH1A2. Conversely, blocking RA synthesis in rabbits impaired their natural ability to regenerate.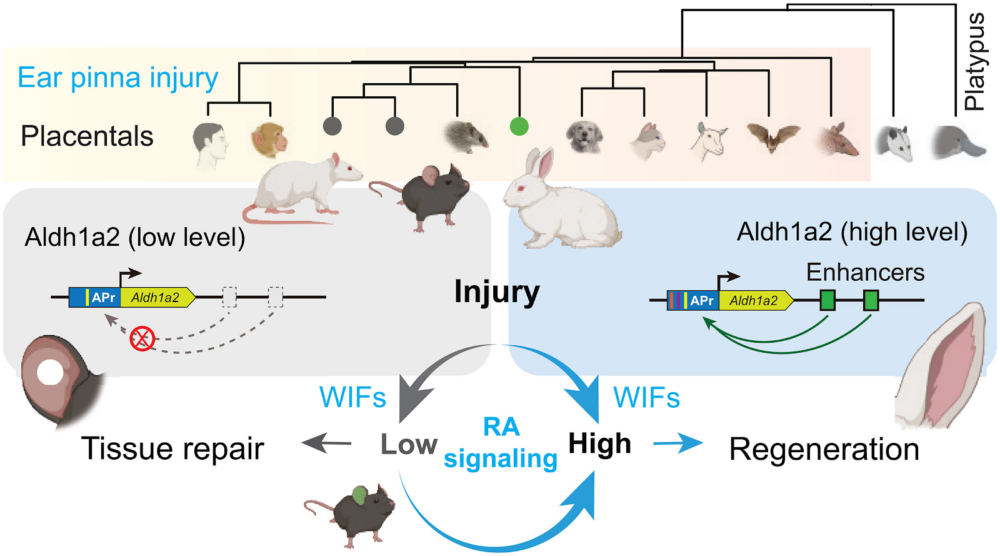
The evolutionary trade-off
The investigation then shifted from a biological question to an evolutionary one: why did mice and rats lose the ability to activate Aldh1a2 during evolution? The researchers found that rabbits have several active enhancers that boost Aldh1a2 transcription after injury. While the DNA for these enhancers exists in mice and rats, it has accumulated mutations over time, rendering them non-functional.
Finally, the researchers created a line of transgenic mice by inserting a single functional rabbit enhancer into the mouse genome to control the mouse’s own Aldh1a2 gene. Reactivation of the RA pathway “transformed the nonregenerating response into a rabbit-like response and directed WIFs to form new tissues,” the paper states.
The authors propose an interesting evolutionary hypothesis as to why the ability to activate Aldh1a2 following injury was lost in some mammalian species. The retinoic acid pathway has multiple jobs. It is crucial both for regeneration and for the normal development and function of sensory systems, particularly hearing and vision.
The authors suggest that the need to build a highly specialized organ, like a high-performance ear, can create an evolutionary trade-off with the ability to regenerate it. In some lineages, like mice and rats, the genetic changes required to tightly control developmental pathways for building this complex ear resulted in the permanent disabling of those same pathways for regeneration after injury.
This might be a common theme in mammals. As the authors note, “recent evidence also suggests that the acquisition of endothermy and the metabolic shift from glycolysis to fatty acid oxidation contributed to cardiomyocyte cell-cycle arrest in adult mammals incapable of heart regeneration.” [2]
By identifying the dormant RA pathway as a master switch, this study provides a clear and actionable target for regenerative medicine. It suggests that reactivating these latent abilities in human tissues may one day be a practical therapeutic strategy.
Literature
[1] Lin, W., Jia, X., Shi, X., He, Q., Zhang, P., Zhang, X., … & Wang, W. (2025). Reactivation of mammalian regeneration by turning on an evolutionarily disabled genetic switch. Science, 388(6754), eadp0176.
[2] Hirose, K., Payumo, A. Y., Cutie, S., Hoang, A., Zhang, H., Guyot, R., … & Huang, G. N. (2019). Evidence for hormonal control of heart regenerative capacity during endot1acquisition. Science, 364(6436), 184-188.









































