In Aging Cell, a team of researchers has described how the health of skin fibroblasts relates to physical and functional ability.
Frailty, capacity, and skin cells
This paper begins with an explanation of how frailty and capacity are defined. The World Health Organization defines it as an overall state of reduced strength, endurance, and physiogical functions that increase the risk of adverse health outcomes and lead to dependency [1]. Intrinsic capacity, while difficult to measure, refers to a collection of functional attributes that define overall health [2]. These metrics lead to a better understanding of how generally healthy someone is than the simple number of chronological age.
However, there is a gap between other clocks, such as epigenetic clocks, and biomarkers of functionality. The authors of this paper focus on a ‘gerophysical’ approach that links the two [3].
This study focuses on skin cells (fibroblasts) for several good reasons. Skin, of course, is easy to measure compared to other parts of the body, and fibroblasts preserve both the function and structure of multiple tissue types [4]. Previous work has found that fibroblasts play a significant role in immune responses [5] and metabolic regulation [6]. These cells have been heavily studied in the context of aging, including epigenetics and transcriptomics such as age-related mRNA strands [7]. However, these researchers note that no one had previously linked cellular aging biomarkers to intrinsic capacity metrics.
Some biomarkers are more telling than others
This study used skin samples from 133 volunteers of both sexes in the INSPIRE-T cohort. Their ages ranged from 20 to 96, encompassing healthy, pre-frail, and frail states.
The first part of this study cultured these fibroblasts in vitro, comparing chronological age to various biomarkers. As expected, the proliferation rate of the fibroblasts slowed with age, and markers of DNA damage increased along with the senescence marker p16 and the inflammatory factor IL-6. Interestingly, this study did not show any statistically significant correlation between chronological age and many other senescence markers, including SA-β-gal. However, older cells did express more SA-β-gal when exposed to the stressor doxorubicin.
In the next part of this study, the researchers focused on three key aspects of fibroblast function: tissue structure, immune responses, and metabolic regulation (SIM) along with senescence. This was a biomarker-based analysis, focusing on several key biomarkers in each of these domains: 31 in total.
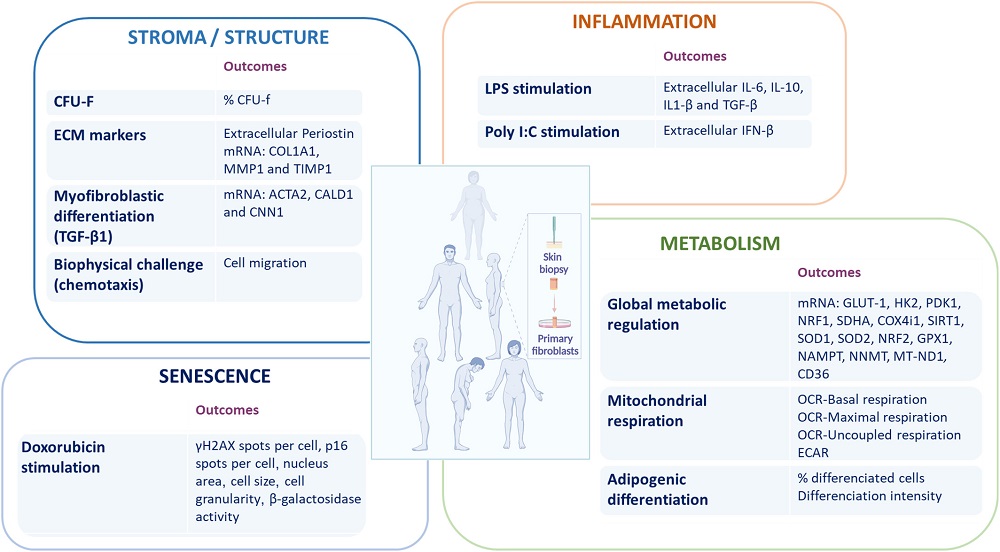
The researchers then used a statistical measurement called Mahalanobis distance to quantify homeostatic dysregulation: how different a person’s biomarkers are from a normal baseline. As expected, this measurement was strongly correlated with chronological age, and the researchers suggest that it can be used as a biomarker of aging.
The researchers took a closer look at how the S, I, and M indices correlate with one another. They found that while many aspects of structure, such as MMP1, did not seem to be closely related with aging, others did, including Periostin and TIMP1. The researchers suggest that these metrics are closely tied to age-related changes in the extracellular matrix.
Cytokine production, as expected, is significantly upregulated with aging, including IL-6. Other well-known inflammatory compounds, such as TGF-β, were undetectable in most samples. Interestingly, aging increases the responsiveness of inflammation to viral infection, although this inflammation’s effect can be negative.
The examination of metabolism revealed a decrease in mitochondrial respiratory efficiency with aging, and antioxidant genes were found to be largely upregulated in response to increased oxidative stress. Once more, there were some interesting negative results: SIRT1 and the NRF genes were not found significantly correlated with aging in this study.
Putting it all together
Directly comparing each of these biomarkers to intrinsic capacity yielded crucial findings. Periostin was once more singled out as a significant contributor to functional aging, as were CD36 and mitochondrial respiration markers. The researchers believe that their overall SIM analysis allows for a holistic approach that provides a detailed analysis of multiple aspects of aging.
Periostin is known in the literature as being crucial to wound healing [8], but it has been little discussed in the context of aging. While the researchers do not directly suggest that it is a suitable target for future interventions, and they cannot demonstrate that it has a causal relationship in this paper, later work may investigate whether or not it is a valid target.
The researchers note this study’s limitations: there were a substantial number of analyses made from a limited number of skin cells, and the biopsies may have been poorly representative of the people from whom they were taken. External factors such as lifestyle and environment could not be accounted for. Larger cohorts would need to be utilized to further refine this SIM analysis.
Literature
[1] Beard, J. R., Officer, A., De Carvalho, I. A., Sadana, R., Pot, A. M., Michel, J. P., … & Chatterji, S. (2016). The World report on ageing and health: a policy framework for healthy ageing. The lancet, 387(10033), 2145-2154.
[2] Gonzalez-Bautista, E., & Beard, J. R. (2023). The Challenge of Measuring Intrinsic Capacity. The journal of nutrition, health & aging, 27(10), 806-807.
[3] Kemoun, P. H., Ader, I., Planat-Benard, V., Dray, C., Fazilleau, N., Monsarrat, P., … & Casteilla, L. (2022). A gerophysiology perspective on healthy ageing. Ageing research reviews, 73, 101537.
[4] Plikus, M. V., Wang, X., Sinha, S., Forte, E., Thompson, S. M., Herzog, E. L., … & Horsley, V. (2021). Fibroblasts: Origins, definitions, and functions in health and disease. Cell, 184(15), 3852-3872.
[5] Haniffa, M. A., Wang, X. N., Holtick, U., Rae, M., Isaacs, J. D., Dickinson, A. M., … & Collin, M. P. (2007). Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. The Journal of Immunology, 179(3), 1595-1604.
[6] Zhao, X., Psarianos, P., Ghoraie, L. S., Yip, K., Goldstein, D., Gilbert, R., … & Liu, F. F. (2019). Metabolic regulation of dermal fibroblasts contributes to skin extracellular matrix homeostasis and fibrosis. Nature metabolism, 1(1), 147-157.
[7] Tsitsipatis, D., Martindale, J. L., Mazan‐Mamczarz, K., Herman, A. B., Piao, Y., Banskota, N., … & Gorospe, M. (2023). Transcriptomes of human primary skin fibroblasts of healthy individuals reveal age‐associated mRNAs and long noncoding RNAs. Aging Cell, 22(11), e13915.
[8] Elliott, C. G., Wang, J., Guo, X., Xu, S. W., Eastwood, M., Guan, J., … & Hamilton, D. W. (2012). Periostin modulates myofibroblast differentiation during full-thickness cutaneous wound repair. Journal of cell science, 125(1), 121-132.








































