Scientists have created an AI model that stratifies Alzheimer’s patients into subgroups that progress slowly or rapidly. When applied to a real-world failed trial, it revealed a robust effect in the former subgroup [1].
Stratify and conquer
Drugs don’t work for everyone equally. Unfortunately, clinical trials are not always able to account for that, which creates several potential problems: what if a drug shown to be ineffective in a trial actually works for a subset of patients? How do we identify this subset and make sure that a useful treatment does not get discarded?
Scientists have suspected for quite a while that this is the case for some experimental treatments for Alzheimer’s disease (AD). The success rate in AD trials is abysmal. Might this be due to the heterogeneity of the patient population?
In a new study from the University of Cambridge, published in Nature Communications, scientists trained their Predictive Prognostic Model (PPM) on data from the ADNI (Alzheimer’s Disease Neuroimaging Initiative) study in order to differentiate their subgroups. The sample size (256 patients) and the number of parameters (just three: β-amyloid, APOE4, and medial temporal lobe grey matter density), were rather small, but according to the authors, it was enough to achieve 91.1% classification accuracy.
The hidden effect
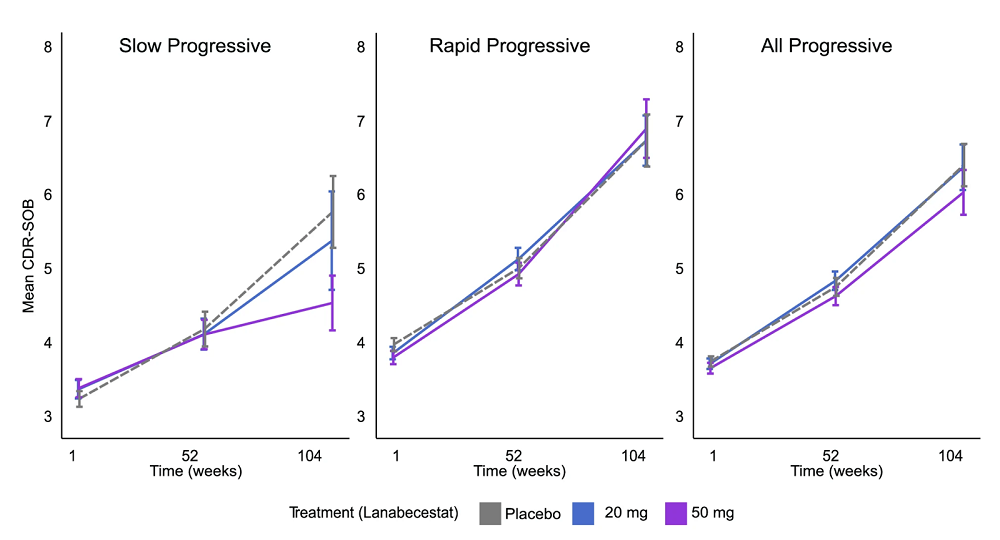
The team then applied their tool to AMARANT, a real-world clinical trial that had failed to show efficacy of lanabecestat, a BACE1 inhibitor designed to reduce the production of β-amyloid plaques in the brain. The AI model analyzed each patient’s baseline data and assigned a prognostic score with which to judge the progression of Alzheimer’s.
“Our AI model gives us a score to show how quickly each patient will progress towards Alzheimer’s disease,” said Professor Zoe Kourtzi in the University of Cambridge’s Department of Psychology, senior author of the study. “This allowed us to precisely split the patients in the clinical trial into two groups: slow- and rapid-progressing, so we could look at the effects of the drug on each group.”
When the researchers analyzed the drug’s efficacy for the slow-progressing subgroup, they found a 46% slowdown in the disease’s progression in patients that received the higher 50mg dose. This is significantly more than what the best treatments that passed their trials achieved for the entire patient population. When the researchers lumped the subgroups back together, the cognitive benefit disappeared, confirming the original outcome and proving that the effect was masked by the heterogeneity of the initial trial population.
Alzheimer’s escape velocity
The team then asked another question: did the treatment prevent patients from transitioning from slow progress to more rapid progress? The answer was positive, as high-dose lanabecestat kept patients in the slow-progressing, hence probably more treatable, subgroup for longer. For the placebo group, 60% of “slow progressors” transitioned to “rapid,” while for the 50mg lanabecestat group, only 33.3% did.
This outcome might be highly relevant considering that new treatments for dementia are nearing approval and will likely be more effective in slowly progressing patients. Combined with better early-stage diagnostics, this might create a sort of “Alzheimer’s escape velocity,” when one treatment slows the disease’s progression enough for upcoming treatments to take over.
“AI can guide us to the patients who will benefit from dementia medicines, by treating them at the stage when the drugs will make a difference, so we can finally start fighting back against these cruel diseases,” said Kourtzi. “Making clinical trials faster, cheaper and better, guided by AI, has strong potential to accelerate the discovery of new precise treatments for individual patients, reducing side effects and costs for health care services.”
Cheaper trials
Stratifying patients with this new AI tool from the start might help make Alzheimer’s clinical trials cheaper and likelier to succeed. The researchers calculated that to detect the drug’s effect in an AI-selected slowly progressing group, a future trial would only need 82 patients per group (treatment vs. placebo). In contrast, to find an effect in a mixed group, a trial would require 762 patients per group. This amounts to a 90% reduction in the required sample size, which could save hundreds of millions of dollars and years of time in drug development. This might seem like abandoning the rapid-progressing patient population, but as this study shows, many of them are former “slow progressors.”
“Promising new drugs fail when given to people too late, when they have no chance of benefiting from them,” Kourtzi said. “With our AI model, we can finally identify patients precisely and match the right patients to the right drugs. This makes trials more precise, so they can progress faster and cost less, turbocharging the search for a desperately needed precision medicine approach for dementia treatment.”
Literature
[1] Vaghari, D., Mohankumar, G., Tan, K., Lowe, A., Shering, C., Tino, P., & Kourtzi, Z. (2025). AI-guided patient stratification improves outcomes and efficiency in the AMARANTH Alzheimer’s Disease clinical trial. Nature Communications, 16(1), 1-12.
View the article at lifespan.io








































