Scientists have demonstrated that injecting healthy mitochondria either systematically or directly into the tumor microenvironment boosts the efficiency of a standard anti-cancer therapy [1].
Mitochondria’s dual role in lung cancer
While not the most prevalent type of cancer, lung cancer causes more deaths than any other. Non-small cell lung cancer (NSCLC) accounts for 85% of cases. This is a less aggressive variety but is still deadly in many cases, even when caught early.
Chemotherapy remains the backbone of treatment for advanced NSCLC, but its success is often undermined by two persistent problems: tumor cells’ adaptability and the toxic impact on the immune system. Anti-cancer treatments have also been shown to accelerate aging [2]. A new study from Tongji University School of Medicine and Nantong University in China, published in Cancer Biology & Medicine, suggests a novel way to address both problems by transplanting healthy mitochondria into the tumor environment.
Cells use two major types of energy production. Oxidative phosphorylation (OXPHOS) is facilitated by mitochondria. It is a complex, multi-stage process that takes time and produces many molecules of ATP (the cell’s energy ‘currency’) for every glucose molecule. It requires oxygen and emits CO2 as a byproduct. Glycolysis occurs in the cytoplasm, does not require oxygen, and produces much smaller amounts of ATP for every glucose molecule.
Despite glycolysis being the more ancient and less effective form of energy production, many tumors reprogram cellular metabolism, including mitochondrial function, to suppress OXPHOS and rely more on glycolysis, a shift known as the Warburg effect [3]. This supports rapid growth and contributes to immune evasion by creating a more acidic environment that weakens immune cells.
Those immune cells, especially T cells and natural killer (NK) cells, also depend on mitochondria to perform their tasks. In the harsh tumor microenvironment, cancer cells can even strip mitochondria from incoming immune cells via filament-like tunneling nanotubes, further weakening the immune response. The researchers hypothesized that supplying fresh, functional mitochondria could help on both fronts, restoring metabolic balance in tumor cells to make them more sensitive to chemotherapy and revitalizing immune cells so that they can attack the tumor more effectively.
Mitochondria hurt cancer cells, boost immune cells
The team transplanted mitochondria from energy-rich human heart muscle cells (cardiomyocytes) into NSCLC models, both in vitro and in mice. In vitro, this was done by co-culturing cancer cells with mitochondria, while in vivo, the researchers used two routes: systemic delivery and local delivery via an injection directly into the tumor site.
Mitochondrial transplantation was combined with cisplatin, a DNA-damaging chemotherapy drug that is standard for NSCLC but known for its immunosuppressive side effects. The team compared three major groups: cisplatin alone, mitochondrial transplantation alone, and the combination. In in vivo experiments, subgroups varied by the type (either systemic or systemic plus local) and frequency of mitochondria delivery (either once or twice per week).
In vitro, mitochondrial transplantation by itself did not kill cancer cells. However, when paired with cisplatin, it nearly halved the concentration of cisplatin required to inhibit cell growth by 50% (IC₅₀) from about 12.9 μM to roughly 6.7 μM. Interestingly, systemic delivery exerted a similar, albeit weaker effect. The combination also shifted tumor metabolism back toward OXPHOS, counteracting the Warburg effect. Markers associated with tumor aggressiveness and therapy resistance, including HIF-1α, CD44, and CD133, were all reduced.
In mice injected with NSCLC cells, the combination treatment significantly slowed tumor growth, with the best results achieved in mice that received both local and systemic mitochondria delivery twice a week. Interestingly, systemic delivery was almost as effective.
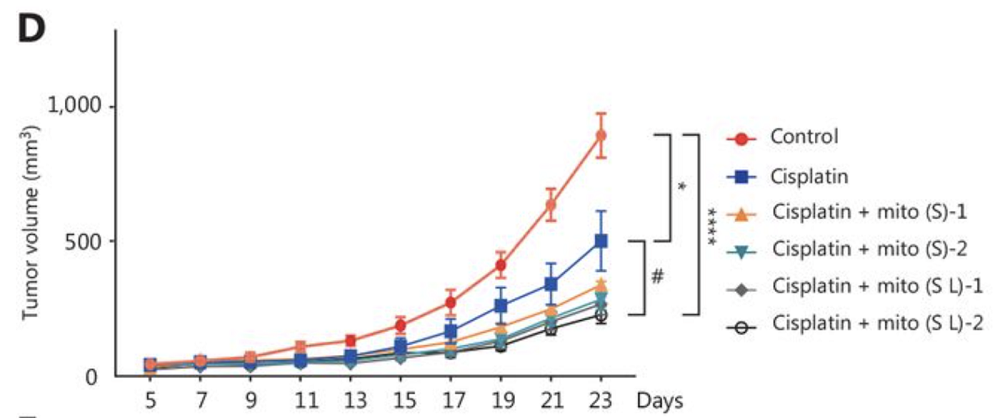
With either method of delivery, tumor stemness/aggressiveness markers such as HIF-1α, CD44, and CD133 were decreased, while markers of programmed cellular death (apoptosis) in cancer cells were increased. Additionally, there was a considerable increase in reactive oxygen species (ROS) in cancer cells. These results suggest that even though the systemic immune boost due to the immune mitochondria uptake is probably a big part of the effect, the tumor cells also end up ingesting those mitochondria, which pushes them metabolically and structurally toward greater vulnerability.
Relevance for future anti-aging treatments
“This research introduces a powerful dual-action strategy,” said Dr. Liuliu Yuan, lead investigator of the study. “By replenishing immune cells with functional mitochondria, we are not just enhancing their energy but restoring their ability to fight. At the same time, tumor cells become more vulnerable to chemotherapy. It’s like rearming the immune system while disarming the tumor. This could be a promising avenue for patients who don’t respond well to conventional treatment.”
As promising as the results are, they come from early-stage research. The delivery method for mitochondrial transplantation, its durability, and its effects in the complex physiology of human cancers will all require further testing. Scaling up mitochondrial production and ensuring consistent quality will also be practical hurdles. However, if mitochondrial transplantation is mastered, it can have implications far beyond anti-cancer treatments, particularly for future anti-aging therapies.
Literature
[1] Lin, S., Yuan, L., Chen, X., Chen, S., Wei, M., Hao, B., … & Fan, L. (2025). Mitochondrial transplantation sensitizes chemotherapy to inhibit tumor development by enhancing anti-tumor immunity. Cancer Biology & Medicine.
[2] Shafqat, S., Chicas, E. A., Shafqat, A., & Hashmi, S. K. (2022). The Achilles’ heel of cancer survivors: fundamentals of accelerated cellular senescence. The Journal of Clinical Investigation, 132(13).
[3] Potter, M., Newport, E., & Morten, K. J. (2016). The Warburg effect: 80 years on. Biochemical Society Transactions, 44(5), 1499-1505.
View the article at lifespan.io








































