Researchers have discovered that infrared lasers promote the clearance of toxic metabolites from the brains of age-accelerated mice by improving lymphatic drainage.
Gunking up the works
Advanced glycation end-products (AGEs), like their acronym suggests, accumulate with age. These substances, which are formed when sugars bind to other molecules without the assistance of enzymes, drive multiple aspects of aging and can lead to metabolic diseases [1]. Introducing AGEs into the brain increases oxidation and leads to amyloid formation [2], and inhibiting the effects of AGEs is a potential treatment method [3].
Previous work has found that many other potentially hazardous, naturally produced substances, including amyloids, are drained by meningeal lymphatic vessels (MLVs) [4]. With aging, MLVs lose their youthful structure, becoming less able to carry away such wastes [5]. The only chemical method that has been found to expand MLVs, however, is vascular endothelial growth factor C, which must be injected directly into the brain in order to work [6].
As the meninges are on the brain’s surface, other treatment methods, such as near-infrared light, are feasible. Previous work has discovered that such phototherapy has beneficial effects in Alzheimer’s model mice [7], and improving MLV drainage also helps to heal brain injury in mice [8]. However, such previous work did not target AGEs, which are the focus of this study.
An age-accelerated model
This study began with ordinary, wild-type Black 6 mice that were exposed to D-galactose (D-gal), which is known to cause oxidative stress, mitochondrial dysfunction, and AGE accumulation [9]. At a 1275-nanometer wavelength, the near-infrared lasers used for the phototherapy were able to penetrate the skull. The researchers chose a laser dose of 10 milliwats per square centimeter, which was judged to be strong enough to provide benefits without excessively heating the brain.
Fluorescent imaging found that D-gal exposure did indeed AGEs in the brains of mice. Applying phototherapy moved these AGEs away from the cortex and into the deep cervical lymph nodes to be cleared. Reactive oxygen species, which cause oxidative damage, were also cleared. The treated mice had much less caspase-3, a compound that leads to cellular death by apoptosis.
The microglia were also significantly affected. Treated animals had considerable reductions in activated microglia, suggesting far less neuroinflammation, and the levels of related inflammatory cytokines were reduced. They also had significantly less astrocyte hypertrophy, signaling a significantly reduced effect of D-gal.
The researchers looked into potential mechanisms behind this clearance. They found that nitric oxide was a key mediator of the process; while MLVs treated with the lasers would expand and allow more clearance, inhibiting the function of nitric oxide prevented this from happening.
Behavioral benefits
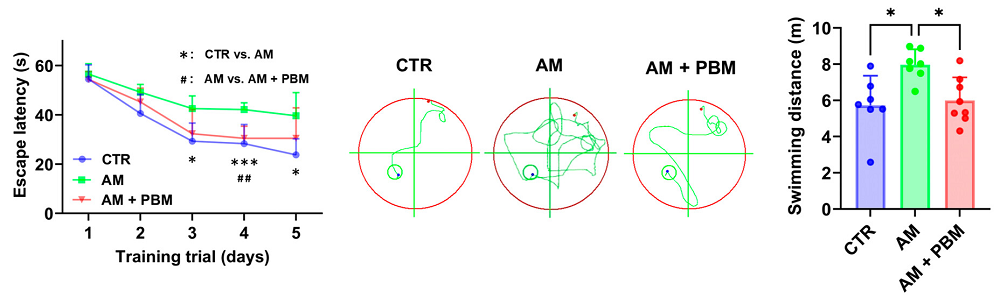
Behavioral testing demonstrated that phototherapy had significant results on the mice’s cognition. In the well-known Morris water maze test, the treated animals’ ability to escape the maze was similar to that of animals that had never been exposed to D-gal at all. This was entirely due to cognition, not motor function. A correlation analysis found that the less oxidative stress and fewer inflammatory cytokines an animal had, the more likely it was to perform well in this test. There were also improvements in the treated mice’s ability to recognize novel objects.
While there are obvious differences between humans and mice that might complicate such an approach, such as brain complexity and skull thickness, phototherapy has been used in human beings before. The researchers point to a study in which four football players with brain damage had been given such a therapy with positive results [10]. Therefore, they believe that this approach “may serve as a safe and effective intervention with high potential for rapid implementation into clinical antiaging applications.”
Literature
[1] Chaudhuri, J., Bains, Y., Guha, S., Kahn, A., Hall, D., Bose, N., … & Kapahi, P. (2018). The role of advanced glycation end products in aging and metabolic diseases: bridging association and causality. Cell metabolism, 28(3), 337-352.
[2] Ko, S. Y., Lin, Y. P., Lin, Y. S., & Chang, S. S. (2010). Advanced glycation end products enhance amyloid precursor protein expression by inducing reactive oxygen species. Free Radical Biology and Medicine, 49(3), 474-480.
[3] Lu, J., Wu, D. M., Zheng, Y. L., Hu, B., Zhang, Z. F., Ye, Q., … & Wang, Y. J. (2010). Ursolic acid attenuates D-galactose-induced inflammatory response in mouse prefrontal cortex through inhibiting AGEs/RAGE/NF-κB pathway activation. Cerebral Cortex, 20(11), 2540-2548.
[4] Dupont, G., Iwanaga, J., Yilmaz, E., & Tubbs, R. S. (2020). Connections between amyloid beta and the meningeal lymphatics as a possible route for clearance and therapeutics. Lymphatic research and biology, 18(1), 2-6.
[5] Da Mesquita, S., Louveau, A., Vaccari, A., Smirnov, I., Cornelison, R. C., Kingsmore, K. M., … & Kipnis, J. (2018). Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature, 560(7717), 185-191.
[6] Da Mesquita, S., Papadopoulos, Z., Dykstra, T., Brase, L., Farias, F. G., Wall, M., … & Kipnis, J. (2021). Meningeal lymphatics affect microglia responses and anti-Aβ immunotherapy. Nature, 593(7858), 255-260.
[7] Li, D., Lin, H., Sun, S., Liu, S., Liu, Z., He, Y., … & Zhu, D. (2023). Photostimulation of lymphatic clearance of β-amyloid from mouse brain: a new strategy for the therapy of Alzheimer’s disease. Frontiers of optoelectronics, 16(1), 45.
[8] Dong, H., Dai, X., Zhou, Y., Shi, C., Bhuiyan, P., Sun, Z., … & Jin, W. (2024). Enhanced meningeal lymphatic drainage ameliorates lipopolysaccharide-induced brain injury in aged mice. Journal of Neuroinflammation, 21(1), 36.
[9] Azman, K. F., & Zakaria, R. (2019). D-Galactose-induced accelerated aging model: an overview. Biogerontology, 20(6), 763-782.
[10] Naeser, M. A., Martin, P. I., Ho, M. D., Krengel, M. H., Bogdanova, Y., Knight, J. A., … & Koo, B. (2023). Transcranial photobiomodulation treatment: significant improvements in four ex-football players with possible chronic traumatic encephalopathy. Journal of Alzheimer’s Disease Reports, 7(1), 77-105.
View the article at lifespan.io








































