Scientists have developed a novel multicellular integrated brain model to study neurological diseases, incorporating all six major brain cell types derived from patient-specific induced pluripotent stem cells (iPSCs) [1].
Beyond organoids
Organoids, tiny lab-grown patches of tissue, are a promising tool for accelerating medical research [2]. They sit a step above cultured cells, being able to mimic the workings of a particular organ such as the liver. Organoids can often replace animal models as a cheaper and more humane alternative.
However, some organs and tissues are harder to replicate than others. Creating brain organoids, for instance, has been a challenge. In a new study published in the Proceedings of the National Academy of Science, MIT scientists have reported a breakthrough: they have created brain organoids called miBrains that contain all the essential brain cell types and even the blood-brain barrier.
Making a piece of brain
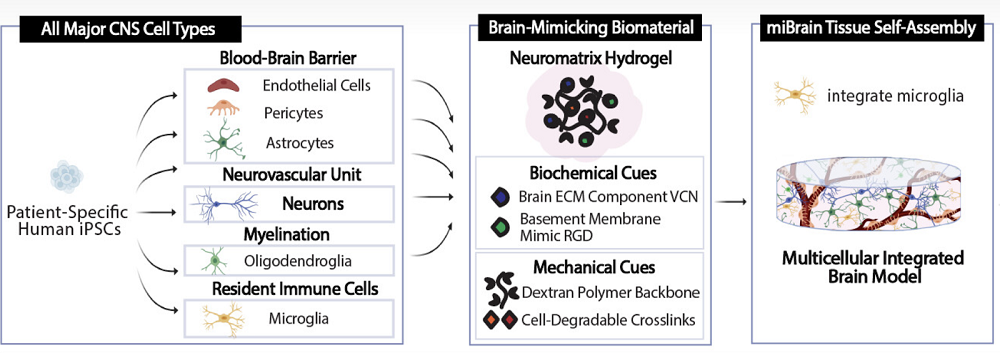
The scientists reprogrammed patient-derived induced pluripotent stem cells (iPSCs) into the required cell types (neurons, astrocytes, oligodendrocytes, microvascular endothelial cells, pericytes, and microglia), validating the transformation by measuring gene expression and cellular function. For instance, iPSC-derived neurons demonstrated robust expression of neuron-associated proteins, formed healthy networks, made abundant connections, fired on their own and when stimulated, and reacted to neurological drugs the way real neurons do.
The cells were mixed together inside a hydrogel environment called Neuromatrix, which closely matches the natural extracellular matrix (ECM), including ECM proteins and RGD, a basement membrane peptide mimic. The researchers experimented with several matrix variants until arriving at the one that the cells were willing to call home.
Vascularizing organoids presents a particular challenge. The brain completely relies on the blood-brain barrier, which consists of endothelial cells in the blood vessels that are tuned to only let certain molecules into the brain microenvironment. The researchers observed the self-assembly of microvessels and the appearance of tight junctions (protein “seals” between adjacent cells that block leaks and set cell polarity) – both crucial BBB features.
Interestingly, miBrain cells were more “lifelike” than their monocultured counterparts. For instance, these astrocytes exhibited enhanced identity and functional gene expression compared to monoculture conditions, and their gene expression was more similar to, but not quite the same as, astrocytes found in the human brain.
A research platform and more
The researchers then conducted an experiment to demonstrate their model’s research value. APOE4, an allele of the gene APOE, is the strongest common genetic risk factor for late-onset Alzheimer’s [3]. Astrocytes make a lot of the APOE protein, but the extent to which astrocytic APOE4 drives pathology is unclear. With miBrain, the researchers were able to swap genotypes cell-by-cell to test whether APOE4 astrocytes are sufficient to trigger downstream problems, and whether this requires microglia crosstalk.
First, they confirmed that miBrains where all cell lineages were carrying APOE4 showed more amyloid aggregation and more tau phosphorylation than controls expressing APOE3 (the “neutral” allele). This signaled that the model naturally expresses APOE4-linked features seen in Alzheimer’s disease. When microglia were removed, the widespread neuronal tau phosphorylation largely dropped, implying the effect depends on astrocyte-to-microglia signaling.
The team then built APOE3 miBrains but replaced only the astrocytes with APOE4 astrocytes using CRISPR. The experiment showed that tau phosphorylation increased in this model, too, indicating that astrocytes can be sufficient to push neuronal tau changes.
“The miBrain is the only in vitro system that contains all six major cell types that are present in the human brain,” said Li-Huei Tsai, director of The Picower Institute of Learning and Memory and senior author of the study. “In their first application, miBrains enabled us to discover how one of the most common genetic markers for Alzheimer’s disease alters cells’ interactions to produce pathology. I’m most excited by the possibility to create individualized miBrains for different individuals. This promises to pave the way for developing personalized medicine.”
“Its highly modular design sets the miBrain apart, offering precise control over cellular inputs, genetic backgrounds, and sensors – useful features for applications such as disease modeling and drug testing,” said Alice Stanton, assistant professor at Harvard Medical School and Massachusetts General Hospital, who co-led the study. “Given its sophistication and modularity, there are limitless future directions. Among them, we would like to harness it to gain new insights into disease targets, advanced readouts of therapeutic efficacy, and optimization of drug delivery vehicles.”
Beyond their obvious uses in studying neurodegenerative diseases, miBrain-like technologies may one day power gradual brain tissue replacement in the longevity context, a project that Jean Hebert is working on at ARPA-H.
Literature
[1] Stanton, A. E., Bubnys, A., Agbas, E., James, B., Park, D. S., Jiang, A., … & Tsai, L. H. (2025). Engineered 3D immuno-glial-neurovascular human miBrain model. Proceedings of the National Academy of Sciences, 122(42), e2511596122.
[2] Yao, Q., Cheng, S., Pan, Q., Yu, J., Cao, G., Li, L., & Cao, H. (2024). Organoids: development and applications in disease models, drug discovery, precision medicine, and regenerative medicine. MedComm, 5(10), e735.
[3] Belloy, M. E., Andrews, S. J., Le Guen, Y., Cuccaro, M., Farrer, L. A., Napolioni, V., & Greicius, M. D. (2023). APOE genotype and Alzheimer disease risk across age, sex, and population ancestry. JAMA neurology, 80(12), 1284-1294.
View the article at lifespan.io








































