A review in Aging Cell has cataloged the harmful effects of EDA2R, a protein that affects three distinct inflammation-related pathways.
A necessary protein gone bad
Like nearly every other protein with documented harmful effects, this one is required for certain systems to function properly. The EDA gene is needed for proper skin development [1] and hair follicle creation [2], and animals with mutated forms of this gene suffer from ectodermal dysplasia, which causes severe malformations in these areas [3].
However, this gene is a member of the tumor necrosis factor (TNF) superfamily [4], which provides a hint as to its potential undesirable effects; TNF-α is a well-known inflammatory biomarker. As previous work has linked EDA2R to muscle atrophy in cancer [5] and some evidence has already suggested that it may be a biomarker of aging [6], these reviewers have gone through the literature to determine what this gene and its protein are doing.
Beyond the skin
As expected, initial work on EDA2R has found that this gene is expressed in the hair and skin [7]. Further work found that it is expressed in the fat, vasculature, and immune system [8], and a comprehensive tissue expression database found that it is highly expressed in the reproductive and endocrine systems along with many other organs. This gene is located close to androgen-related genes on the X chromosome, so it is unsurprising that it has related effects involving male pattern baldness [9] and that its tissue expression varies between men and women [10]; that particular study also pinpointed EDA2R as a potential biomarker of aging. Furthermore, and perhaps more crucially, its genetic locus has been found to be associated with lipid and lipoprotein metabolism [11].
There are three different pathways by which EDA2R appears to lead to inflammaging. Through TRAF6, the well-known canonical NF-κB pathway and the JNK pathway can be activated, but a different ligand, TRAF3, activates the non-canonical NF-κB pathway. All three of these pathways have been documented to be involved in inflammation [12-14].
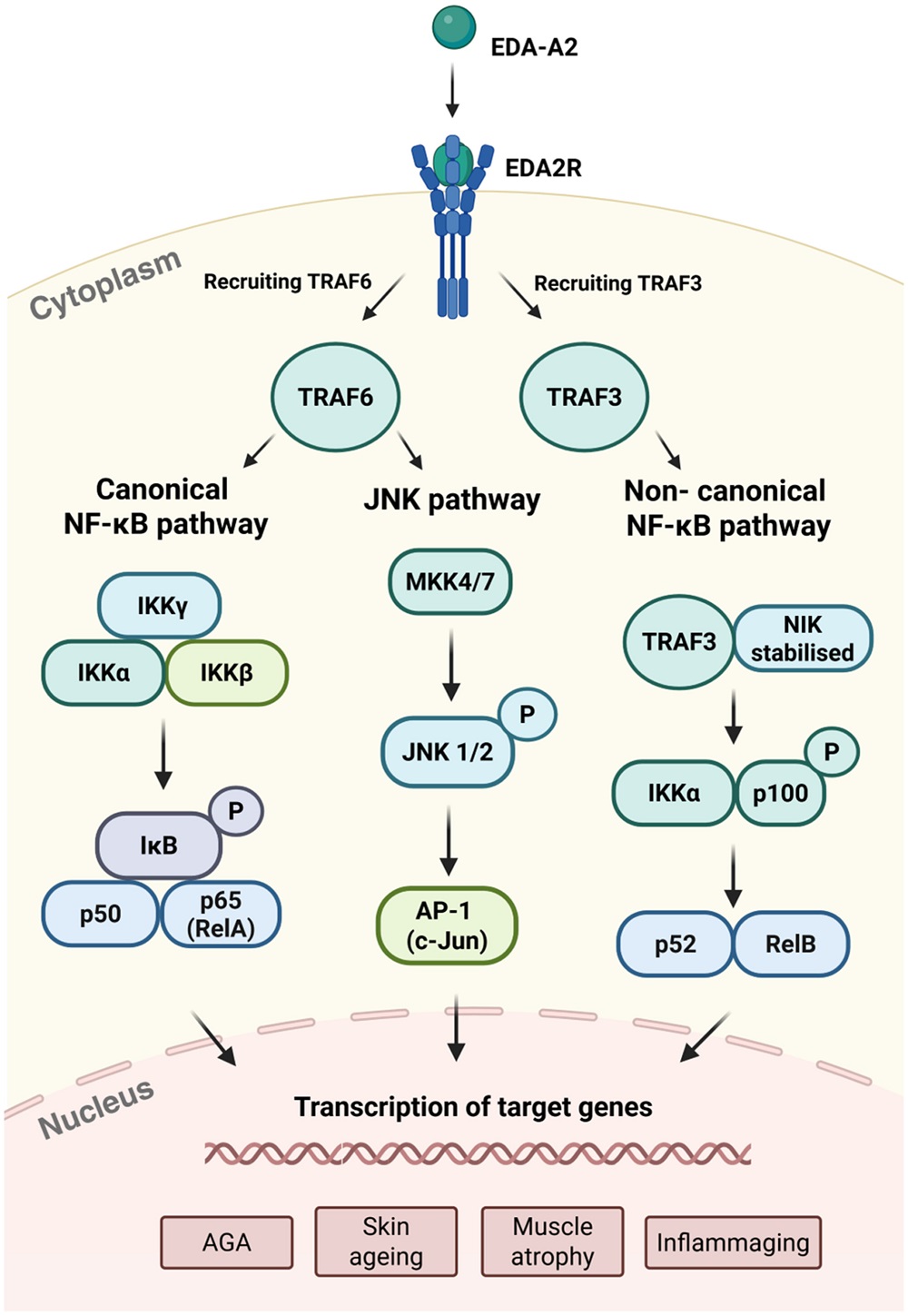
There have been several studies suggesting a direct relationship between EDA2R and both inflammatory and age-related diseases. Overexpression of EDA2R is associated with an overexpression of lipids related to acne [15], and it is increased in progeria as well [10]. Another study found that its overexpression is related to many other lipid-related disorders, including characteristics of obesity along with metabolic issues; that study also found a related increase in inflammation [16]. There has also been a documented, significant relationship between EDA2R and frailty [17].
The researchers sum up these findings: “Collectively, the evidence from diverse cohorts, animal models and clinical studies underscores EDA2R gene expression as a robust and versatile biomarker of multiple interconnected pathophysiological processes.”
Further work appears to agree with this assessment. One study that used UK Biobank data found that the EDA2R protein is associated with premature aging, including both frailty and multiple epigenetic aging clocks, such as PhenoAge, along with having a negative healthspan association [18]. A different study found it to be associated with a broad range of cancers [19], another study found it to be linked to metabolic disorders such as diabetes [20], and yet another found a link between EDA2R and dementia [21].
A potentially difficult target
The reviewers took some time to discuss the physical intricacies of EDA2R as a protein, including its shape and the protein it binds to. They note its similarities to EDAR and that there are only two amino acid residues that distinguish the two, which may make it more difficult to develop a drug that targets EDA2R without affecting EDAR. The protein’s folded structure has also not been conclusively determined, although AI-based systems such as AlphaFold have made good guesses and it is possible to use expensive but slow microscopy techniques to have a definitive answer.
However, there are some existing techniques that appear to decrease EDA2R. A mouse study found that ginkgolide B decreases the expression of the gene’s murine counterpart [22], while human studies have found that excessive bed rest increases it [23] while fasting decreases it [24]. This suggests that it may be modulated by the same general treatment recommended everywhere: diet and exercise.
Literature
[1] Mikkola, M. L. (2008). TNF superfamily in skin appendage development. Cytokine & growth factor reviews, 19(3-4), 219-230.
[2] Lee, J., & Tumbar, T. (2012, October). Hairy tale of signaling in hair follicle development and cycling. In Seminars in cell & developmental biology (Vol. 23, No. 8, pp. 906-916). Academic Press.
[3] Katthika, V. K., & Auerkari, E. I. (2018, May). Ectodermal Dysplasia. In 11th International Dentistry Scientific Meeting (IDSM 2017) (pp. 230-238). Atlantis Press.
[4] Dostert, C., Grusdat, M., Letellier, E., & Brenner, D. (2019). The TNF family of ligands and receptors: communication modules in the immune system and beyond. Physiological reviews, 99(1), 115-160.
[5] Bilgic, S. N., Domaniku, A., Toledo, B., Agca, S., Weber, B. Z., Arabaci, D. H., … & Kir, S. (2023). EDA2R–NIK signalling promotes muscle atrophy linked to cancer cachexia. Nature, 617(7962), 827-834.
[6] Arif, M., Lehoczki, A., Haskó, G., Lohoff, F. W., Ungvari, Z., & Pacher, P. (2025). Global and tissue-specific transcriptomic dysregulation in human aging: Pathways and predictive biomarkers. GeroScience, 1-20.
[7] Bergqvist, C., Ramia, P., Abbas, O., & Kurban, M. (2017). Genetics of syndromic and non‐syndromic hereditary nail disorders. Clinical Genetics, 91(6), 813-823.
[8] Kanoni, S., Graham, S. E., Wang, Y., Surakka, I., Ramdas, S., Zhu, X., … & Leonard, H. L. (2022). Implicating genes, pleiotropy, and sexual dimorphism at blood lipid loci through multi-ancestry meta-analysis. Genome biology, 23(1), 268.
[9] Prodi, D. A., Pirastu, N., Maninchedda, G., Sassu, A., Picciau, A., Palmas, M. A., … & Pirastu, M. (2008). EDA2R is associated with androgenetic alopecia. Journal of Investigative Dermatology, 128(9), 2268-2270.
[10] Barbera, M. C., Guarrera, L., Re Cecconi, A. D., Cassanmagnago, G. A., Vallerga, A., Lunardi, M., … & Bolis, M. (2025). Increased ectodysplasin-A2-receptor EDA2R is a ubiquitous hallmark of aging and mediates parainflammatory responses. Nature Communications, 16(1), 1898.
[11] Zoodsma, M., Beuchel, C., Yasmeen, S., Kohleick, L., Nepal, A., Koprulu, M., … & Langenberg, C. (2025). A genetic map of human metabolism across the allele frequency spectrum. Nature Genetics, 1-11.
[12] Moneva-Sakelarieva, M., Kobakova, Y., Konstantinov, S., Momekov, G., Ivanova, S., Atanasova, V., … & Atanasov, P. (2025). The role of the transcription factor NF-kB in the pathogenesis of inflammation and carcinogenesis. Modulation capabilities. Pharmacia, 72, 1-13.
[13] Wu, N., Wang, S., Zhang, Y., & Wang, S. (2025). Research Progress on Anti-Inflammatory Mechanism of Inula cappa. International Journal of Molecular Sciences, 26(5), 1911.
[14] Kaltschmidt, C., Greiner, J. F., & Kaltschmidt, B. (2021). The transcription factor NF-κB in stem cells and development. Cells, 10(8), 2042.
[15] Kwack, M. H., Hamida, O. B., Lee, W. J., & Kim, M. K. (2024). EDA-A2 increases lipid production in EDA2R-expressing human sebocytes. Journal of Dermatological Science, 113(1), 34-37.
[16] Arif, M., Lehoczki, A., Haskó, G., Lohoff, F. W., Ungvari, Z., & Pacher, P. (2025). Global and tissue-specific transcriptomic dysregulation in human aging: Pathways and predictive biomarkers. GeroScience, 1-20.
[17[ Perez, K., Ciotlos, S., McGirr, J., Limbad, C., Doi, R., Nederveen, J. P., … & Melov, S. (2022). Single nuclei profiling identifies cell specific markers of skeletal muscle aging, frailty, and senescence. Aging (Albany NY), 14(23), 9393.
[18] Ma, L. Z., Liu, W. S., He, Y., Zhang, Y., You, J., Feng, J. F., … & Yu, J. T. (2025). Plasma proteomics identify novel biomarkers and dynamic patterns of biological aging. Journal of Advanced Research.
[19] Papier, K., Atkins, J. R., Tong, T. Y., Gaitskell, K., Desai, T., Ogamba, C. F., … & Travis, R. C. (2024). Identifying proteomic risk factors for cancer using prospective and exome analyses of 1463 circulating proteins and risk of 19 cancers in the UK Biobank. Nature Communications, 15(1), 4010.
[20] Qian, H., Wu, C., Li, B., Rosenzweig, A., & Wang, M. (2025). Plasma Proteomics Linking Primary and Secondary diseases: Insights into Molecular Mediation from UK Biobank Data. medRxiv, 2025-08.
[21] Gong, J., Williams, D. M., Scholes, S., Assaad, S., Bu, F., Hayat, S., … & Steptoe, A. (2025). Unraveling the role of proteins in dementia: insights from two UK cohorts with causal evidence. Brain Communications, 7(2), fcaf097.
[22] Lee, C. W., Wang, B. Y. H., Wong, S. H., Chen, Y. F., Cao, Q., Hsiao, A. W. T., … & Lee, O. K. S. (2025). Ginkgolide B increases healthspan and lifespan of female mice. Nature aging, 5(2), 237-258.
[23] Fernandez‐Gonzalo, R., Tesch, P. A., Lundberg, T. R., Alkner, B. A., Rullman, E., & Gustafsson, T. (2020). Three months of bed rest induce a residual transcriptomic signature resilient to resistance exercise countermeasures. The FASEB Journal, 34(6), 7958-7969.
[24] Pietzner, M., Uluvar, B., Kolnes, K. J., Jeppesen, P. B., Frivold, S. V., Skattebo, Ø., … & Langenberg, C. (2024). Systemic proteome adaptions to 7-day complete caloric restriction in humans. Nature metabolism, 6(4), 764-777.
View the article at lifespan.io








































