Nov 7, 2025 – Insilico Medicine (“Insilico”), a clinical-stage generative artificial intelligence (AI)-driven drug discovery and development company, today announced the launch of its innovative cardiometabolic disease portfolio of unique highly-differentiated molecules discovered using generative AI.
Powered by Insilico’s proprietary end-to-end Pharma.AI platform, the portfolio covers a range of diverse mechanisms and stages from early discovery to preclinical development. It consists of eight oral small molecules with unique properties designed to unlock the full potential of established high-confidence targets such as GLP-1R, GIPR, Amylin, APJ, and Lp(a), as well as moderate-novelty targets such as NLRP3 and NR3C1. The two novel GLP-1RAs are designed for improved safety and pharmacokinetics at low dose to allow for multi-pill combinations with the other molecules in the portfolio. One of the GLP-1RAs is designed to sustain once-weekly (QW) dosing.
“Cardiometabolic and antiobesity drugs such as GLP-1RAs may be the first wave of longevity therapeutics increasing both healthspan and lifespan in a large population. With our cardiometabolic strategy we decided to choose several established and moderate-novelty targets but deliver a high level of differentiation through novelty in chemistry focusing on properties to help unlock the full potential of the targets in a variety of diseases and biologic processes and allow for combinations at low dose. Our multi-parameter optimized GLP-1RAs are oral small molecules with very high level of preclinical safety tested in multiple species and unique molecular structure that can potentially sustain once a week (QW) dosing” said Alex Zhavoronkov, PhD, founder and CEO of Insilico Medicine.
Insilico’s Diverse AI-Powered Pipeline for Cardiometabolic Disease Innovation
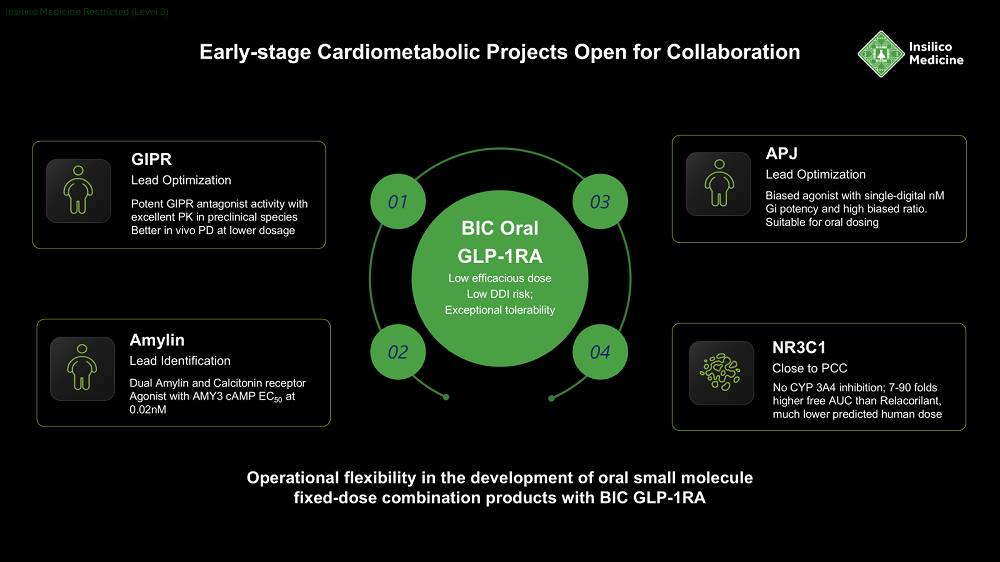
The introduction of Insilico’s new cardiometabolic portfolio, powered by its Pharma.AI platform, features eight programs targeting seven distinct targets, spanning from lead optimization to IND-enabling stages.
At the center of the portfolio’s lineup are two oral small-molecule candidate agonists targeting the GLP-1 receptor (GLP-1RAs), which have reached preclinical candidate (PCC) stage and form the basis of the portfolio’s metabolic effects designed for mono- and combination therapy with the other molecules. Also nearing the PCC stage, a small molecule targeting NR3C1 is designed to treat hypercortisolism-associated metabolic diseases.
In the early stages of the R&D pipeline, four additional programs targeting GIPR, Amylin, APJ, and Lp(a) are currently in the lead optimization phase. AI-driven development has led to significant differentiation and enhanced properties compared to existing therapies.
Rounding out the newly disclosed programs, ISM8969 stands out as the most advanced candidate, having recently completed IND-enabling studies. ISM8969 is a potentially best-in-class, highly selective, orally available, and brain-penetrant small molecule inhibitor of NLRP3, designed primarily for the treatment of inflammatory and central nervous system (CNS) diseases.
For more details, please contact: BD@insilico.com
The portfolio is centered around the following targets:
GLP-1RA (QD); Stage: IND-enabling
This is a fully biased once-daily oral GLP-1R agonist that combines low microsomal clearance with minimal CYP inhibition, favorable cross-species pharmacokinetics, delivering favorable long-term anti-obesity efficacy alongside acute food-intake and glucose-control effects.
GLP-1R (QW); Stage: Pre-Developmental Candidate
This is a fully biased oral GLP-1R agonist engineered for once-weekly dosing, offering high solubility, extremely low metabolic and systemic clearance, long predicted half-life supporting the potential for weekly dosing with weight-loss efficacy comparable or superior to daily existing oral GLP-1R agonist.
NR3C1; Stage: Close to Preclinical Candidate
Insilico’s NR3C1 antagonist is a proprietary, selective glucocorticoid receptor blocker that delivers strong solubility, permeability, and no CYP3A4 inhibition. Improved pharmacokinetic properties compared to other NR3C1 inhibitors, with significantly higher exposure and improved in vivo efficacy, translate into stronger low-dose efficacy in reversing cortisol-induced metabolic dysfunction and enhancing semaglutide-mediated weight loss, positioning it for orphan-eligible Cushing’s syndrome and broader hypercortisolism-linked metabolic diseases.
NLRP3; Stage: IND-enabling
This is an orally bioavailable, brain-penetrant small molecule that selectively and potently inhibits NLRP3, shows a promising in vitro safety/PK profile, and delivers robust efficacy across preclinical models of Parkinson’s disease, peritonitis, pancreatitis, multiple sclerosis, collectively giving it a differentiated edge over largely peripherally restricted competitor compounds.
Dual Amylin and Calcitonin Receptor Agonist; Stage: Lead Identification
This program features a potent dual amylin and calcitonin receptor agonist (DACRA) designed to deliver synergistic metabolic benefits by enhancing satiety, reducing food intake, and improving glycemic control. The molecule exhibits novel structure and high receptor potency.
GIPR; Stage: Lead Optimization
The next-generation GIP receptor (GIPR) antagonist is designed to complement GLP-1–based therapies by enhancing insulin secretion, improving lipid metabolism, and amplifying weight-loss efficacy. Leveraging Insilico’s generative AI design capabilities, the molecule exhibits high predicted receptor selectivity, optimized metabolic stability, and favorable oral bioavailability. It is being advanced to achieve synergistic efficacy and improved tolerability in multi-drug combinations, positioning it as a differentiated candidate for obesity and type 2 diabetes treatment.
APJ; Stage: Lead Optimization
Selective activation of the APJ G-protein signaling pathway yields cardiovascular benefits, reduces inflammation, and improves metabolic profiles. In contrast, simultaneous activation of both G protein and β-arrestin pathways may cause cardiac hypertrophy and increased inflammation, while receptor desensitization can compromise long-term efficacy. Utilizing Insilico’s generative AI and multi-parameter optimization framework, the ISM molecule was designed as a potent and highly biased APJ agonist with novel scaffold, showing promise for enhanced safety and therapeutic outcomes. The lead compound lowered blood glucose, promoted body weight loss, and improved lean mass ratio in DIO mice following oral administration, overcoming limitations associated with non-biased agonists.
Lp(a); Stage: Lead Optimization
This molecule features a novel structure which exhibits superior in vivo pharmacokinetic profiles, with higher AUC and longer half-life in animal studies. It demonstrates comparable in vivo efficacy with the clinically validated Lp(a) lowering candidate achieving a reduction in Lp(a) levels in transgenic mouse models, equivalent to the clinically validated candidate. The safety profile is improved, showing significantly less off-target plasminogen inhibition after 5-day BID dosing.
“The AI revolution in drug discovery is no longer theoretical, as demonstrated by our clinical-stage programs in fibrosis, oncology, and inflammation,” said Feng Ren, PhD, Co-CEO and Chief Scientific Officer of Insilico Medicine. “By harnessing our proven generative AI platform to address the complexity of cardiometabolic disease, we are committed to accelerating the discovery and development of differentiated therapies that will benefit millions of patients worldwide and help people live longer and healthier.”
Setting the Benchmark: Insilico’s Breakthroughs in AI-Powered Therapeutics
Since pioneering next-generation AI in drug discovery, Insilico Medicine has built an extensive therapeutic portfolio across high-demand therapeutic areas, rapidly advancing its internal R&D pipeline and setting a new industry benchmark for efficiency. Traditionally, early-stage drug discovery can take 2.5 to 4 years, while Insilico has nominated 22 preclinical candidates at an average pace of just 12 to 18 months per program, synthesizing and testing only 60 to 200 molecules each, which highlights the exceptional capabilities of its AI-driven platform and expert multidisciplinary validation teams.
Within the company’s clinical-stage programs, Rentosertib, the world’s first AI-discovered novel-mechanism anti-fibrotic candidate, has completed Phase 2a proof-of-concept clinical trial, demonstrating promising efficacy trends and a favorable safety profile. ISM5411, the PHD1/2 inhibitor with best-in-class potential for treating inflammatory bowel disease (IBD) has completed two Phase I trials, showing good safety and a gut-restricted PK profile. Additionally, three of Insilico’s anti-tumor programs have now reached the first-in-patient dosing stage, with interim results expected in the near future.
In recent years, Insilico has steadily increased its investment in cutting-edge research, remaining dedicated to sharing its discoveries through peer-reviewed publications. Since the beginning of 2024, the company has published 6 papers in the Nature portfolio:
- Nature Biotechnology: A small-molecule TNIK inhibitor targets fibrosis in preclinical and clinical models
- Nature Biotechnology: Intestinal mucosal barrier repair and immune regulation with an AI-developed gut-restricted PHD inhibitor
- Nature Biotechnology: Quantum-computing-enhanced algorithm unveils potential KRAS inhibitors
- Nature Communications: A novel, covalent broad-spectrum inhibitor targeting human coronavirus Mpro
- Nature Communications: Oral ENPP1 inhibitor designed using generative AI as next generation STING modulator for solid tumors
- Nature Medicine: A generative AI-discovered TNIK inhibitor for idiopathic pulmonary fibrosis: a randomized phase 2a trial
In March 2024, Insilico published in Nature Biotechnology the journey of the Rentosertib program from inception to Phase 1 clinical trials, along with part of experimental data. In December 2024, Insilico published in Nature Biotechnology the AI-enabled preclinical research journey and partial experimental data for the gut-restricted PHD1/2 inhibitor ISM5411. In January 2025, Insilico, together with partners including the University of Toronto, published research in Nature Biotechnology on exploring generative AI with a quantum-classical hybrid model to design novel KRAS inhibitors. In May 2025, Insilico published collaborative research in Nature Communications on AI-enabled development of pan-coronavirus inhibitors. In the same month, Insilico published another Nature Communications paper on AI-enabled development of next-generation ENPP1 inhibitors for innate immune modulation. Most recently, in June 2025, Insilico reported the Phase IIa clinical result of Rentosertib in a Nature medicine paper.
Leveraging sustained scientific breakthroughs at the intersection of biotechnology, artificial intelligence, and automation, Insilico ranked Top 100 global corporate institutions in Nature Index‘s “2025 Research Leaders: global corporate institutions for biological sciences and natural sciences publications”.
About Insilico Medicine
Insilico Medicine, a leading and global AI-driven biotech company, utilizes its proprietary Pharma.AI platform and cutting-stage automated laboratory to accelerate drug discovery and advance innovations in life sciences research. By integrating AI and automation technologies and deep in-house drug discovery capabilities, Insilico is delivering innovative drug solutions for unmet needs including fibrosis, oncology, immunology, pain, and obesity and metabolic disorders. Additionally, Insilico extends the reach of Pharma.AI across diverse industries, such as advanced materials, agriculture, nutritional products and veterinary medicine.
For more information, visit www.insilico.com.
Media Contact: media@insilicomedicine.com.
View the article at lifespan.io









































