A recent review in the Journal of Ovarian Research summarizes current knowledge of the impact of various polyphenols on different aspects of ovarian aging. The researchers discuss that polyphenol supplementation could be used as an intervention to delay ovarian aging [1].
Every woman’s problem
Ovarian aging and cessation of proper ovarian functioning precede aging in other organs. Ovarian aging is related to a reduction of oocyte quality and quantity, which is the main reason for age-related infertility.
Beyond the fertility problems, ovarian aging impacts lifespan and is also linked to many age-related conditions, such as osteoporosis, cardiovascular disease, and neurodegenerative disorders. Therefore, finding ways to delay it is in the interest of every female, regardless of her childbearing goals.
At this moment, hormone replacement therapy and assisted reproductive technologies are used to address ovarian aging-related problems; however, they are unable to reverse female reproductive aging and the declining ovarian reserve. Longevity researchers are currently seeking ways to extend the female reproductive span, but before effective therapies are on the market, lifestyle factors can be utilized to address ovarian aging.
The authors of this review highlight polyphenols, naturally occurring metabolites found in fruits, vegetables, nuts, seeds, herbs, spices, and medicinal plants, as one possible intervention. Polyphenols exhibit strong biological activities, including antioxidant, anti-inflammatory, antibacterial, and antiviral properties, and demonstrate numerous beneficial effects. Studies also suggest that they can reduce the risk of cardiovascular disease and neurodegenerative disorders [2].
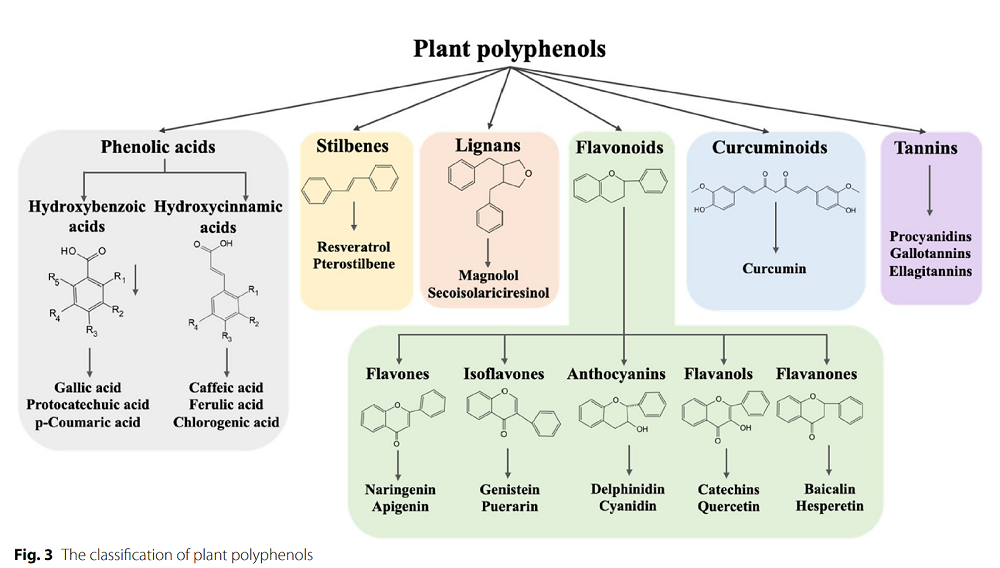
Delaying ovarian aging
The first part of this review discusses resveratrol, a plant-derived polyphenol that has antioxidant and anti-apoptotic properties. Multiple studies have shown that resveratrol’s dietary supplementation or oral administration ”alleviated ovarian oxidative stress damage, restored hormone levels, reduced ovarian cell apoptosis, and improved reproductive performance in animals” [3-7]. It also positively affected epigenetic changes and gene expression in the aging ovary.
Polyphenols extracted from tea leaves were shown to reduce inflammatory responses and oxidative stress and to improve ovarian reserve and ovarian function in animal models of induced ovarian damage [8, 9]. Human, mice, and other mammalian research also suggested their benefits in “alleviating ovarian aging and improving reproductive performance by enhancing oocyte quality and reducing oxidative stress” [10-12].
Quercetin was described as having strong antioxidant properties. It can promote in vitro maturation of oocytes from aged mice and humans [13] and slow down the aging of human ovarian cells [14]. Experiments in middle-aged mice also showed that oral administration improved estrous cycles, pregnancy rate, and ovarian reserve [14]. In polycystic ovary syndrome (PCOS) models, quercetin reversed many detrimental changes, such as irregular ovulation and hormone secretion, or increased ovarian cell apoptosis and inflammation [15, 16]. Similar beneficial effects were also seen in experiments in livestock and poultry animals.
Proanthocyanidins are among the most potent natural antioxidants and possess several other beneficial characteristics. They have been linked to ovarian health in multiple studies. Research in rodents and human cells reported that proanthocyanidins reduced oxidative stress, inhibited ovarian cells apoptosis, improved oocyte viability and quality, modulated hormone levels, and alleviated PCOS [17-20].
Less commonly studied polyphenols also mitigate ovarian aging. Curcumin “has been found to delay the ovarian aging process by increasing follicular number, modulating hormone secretion, reducing oxidative stress, enhancing oocyte maturation and embryo development in an aged mouse model” [21]. Other polyphenols, such as chlorogenic acid, ferulic acid, and pterostilbene, have been shown to positively impact ovarian reserve, ovarian function, and oocyte quality by mitigating oxidative stress, reducing ovarian cell apoptosis, and reducing DNA damage [22-25].
It is worth noting that several studies have shown that a moderate dose of polyphenols can make a profound difference, and high doses of polyphenols can be toxic and cause ovarian damage and impair oocyte maturation. [17, 26-29].
Many mechanisms, one goal
The beneficial effects of polyphenols can be achieved through modulations of many molecular pathways. One of them involves oxidative stress and the excessive production of reactive oxygen species (ROS), which can contribute to an increase in inflammation and disrupt the redox balance, leading to damage to mitochondrial function, telomere shortening, apoptosis, and inflammation, all of which compromise the proper functioning of ovaries and contribute to ovarian aging.
Since polyphenols have antioxidant properties, the researchers investigated their role in delaying ovarian aging by examining the effects of resveratrol, quercetin, and epigallocatechin gallate (EGCG). Those polyphenols have been shown to modulate multiple molecular signaling pathways related to oxidative stress, improve ovarian antioxidant capacity, and reduce ovarian cell apoptosis [30-32]. A human randomized controlled trial also showed a positive role of plant polyphenols (curcumin or resveratrol supplementation) in alleviating ovarian aging by reducing oxidative stress [33, 34].
Polyphenols’ anti-inflammatory properties can also help alleviate ovarian aging by reducing inflammation, which negatively impacts ovarian health. These benefits were demonstrated in animal and human models of ovarian damage [8,35]. Reduction of inflammation and improved oocyte and embryo quality resulted from polyphenol supplementation in women with PCOS who received quercetin [36]. However, there is still a need for research on the impact of polyphenols on inflammation markers in naturally aging ovaries.
Aging-related hormonal dysregulation significantly impacts ovarian aging. The hypothalamic-pituitary-ovary (HPO) axis controls hormones related to the reproductive system. Aging-related dysregulation of the HPO axis contributes to the depletion of the ovarian reserve and decreased quality and quantity of ovarian cells [37]. A growing body of research suggests that plant polyphenols regulate the sensitivity and secretion of reproductive hormones, potentially mitigating ovarian aging. The impact of polyphenols on hormonal balance and ovarian health was tested in preclinical and clinical studies of women with PCOS, showing their benefits for ovarian health through the regulation of hormone secretion [38-40].
The microenvironment surrounding the ovaries is also essential. This environment normally supports ovary and oocyte maturation, but aging leads to the accumulation of metabolites that might disrupt the homeostasis. The gut microbiota and its metabolites influence the ovarian microenvironment via the gut-ovary axis. Through this axis, the ovary communicates with the gut microbiota via hormone secretion. On the other hand, gut microbes produce metabolic signalling molecules that can impact ovarian function. Studies that used fecal microbiota transplants suggest that maintaining “a youthful gut microbiota may help preserve ovarian function and reproductive health” [41]. Similarly, experiments with laying hens as a model suggested that polyphenol supplementation could improve ovarian function by modulating the gut microbiota [27, 42].
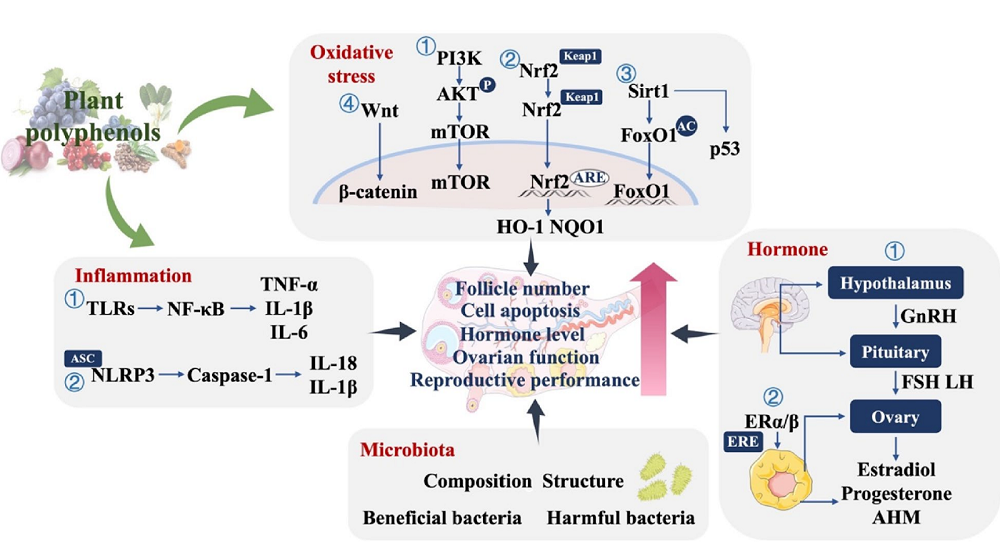
There appear to be many mechanisms and pathways through which polyphenols impact ovarian aging. This is unsurprising since polyphenols are a broad group of molecules with diverse chemical structures, resulting in distinct bioactivity profiles.
This knowledge can be used to design safe plant polyphenol-based interventions for female reproductive longevity that can be used alone or in combination with other treatments.
Literature
[1] Gong, H., Zhang, H., Liu, Y., Mao, X., & Wang, J. (2025). Role and mechanisms of plant polyphenols in ovarian aging. Journal of ovarian research, 18(1), 239.
[2] Potì, F., Santi, D., Spaggiari, G., Zimetti, F., & Zanotti, I. (2019). Polyphenol Health Effects on Cardiovascular and Neurodegenerative Disorders: A Review and Meta-Analysis. International journal of molecular sciences, 20(2), 351.
[3] Yong, W., Jiao, J., Kou, Z., Wang, C., & Pang, W. (2021). Resveratrol ameliorates malathion-induced estrus cycle disorder through attenuating the ovarian tissue oxidative stress, autophagy and apoptosis. Reproductive toxicology (Elmsford, N.Y.), 104, 8–15.
[4] Okamoto, N., Sato, Y., Kawagoe, Y., Shimizu, T., & Kawamura, K. (2022). Short-term resveratrol treatment restored the quality of oocytes in aging mice. Aging, 14(14), 5628–5640.
[5] Wu, H., Xue, J., Di, H., Lv, C., Hao, Y., & Nie, Z. (2022). Resveratrol improves ovarian function in aged rat by inhibiting oxidative stress and activating the Sirt1. General physiology and biophysics, 41(1), 53–61.
[6] Herrero, Y., Velázquez, C., Pascuali, N., May, M., Abramovich, D., Scotti, L., & Parborell, F. (2023). Resveratrol alleviates doxorubicin-induced damage in mice ovary. Chemico-biological interactions, 376, 110431.
[7] Zhu, H., Li, X., Qiao, M., Sun, X., & Li, G. (2023). Resveratrol Alleviates Inflammation and ER Stress Through SIRT1/NRF2 to Delay Ovarian Aging in a Short-Lived Fish. The journals of gerontology. Series A, Biological sciences and medical sciences, 78(4), 596–602.
[8] Fabbri, R., Macciocca, M., Vicenti, R., Caprara, G., Piccinni, M. P., Paradisi, R., Terzano, P., Papi, A., & Seracchioli, R. (2019). Epigallocatechin-3-gallate inhibits doxorubicin-induced inflammation on human ovarian tissue. Bioscience reports, 39(5), BSR20181424.
[9] Chen, Q., Xu, Z., Li, X., Du, D., Wu, T., Zhou, S., Yan, W., Wu, M., Jin, Y., Zhang, J., & Wang, S. (2021). Epigallocatechin gallate and theaflavins independently alleviate cyclophosphamide-induced ovarian damage by inhibiting the overactivation of primordial follicles and follicular atresia. Phytomedicine : international journal of phytotherapy and phytopharmacology, 92, 153752.
[10] Zhang, H., Su, W., Zhao, R., Li, M., Zhao, S., Chen, Z. J., & Zhao, H. (2024). Epigallocatechin-3-gallate improves the quality of maternally aged oocytes. Cell proliferation, 57(4), e13575.
[11] Fan, Z., Xiao, Y., Chen, Y., Wu, X., Zhang, G., Wang, Q., & Xie, C. (2015). Effects of catechins on litter size, reproductive performance and antioxidative status in gestating sows. Animal nutrition (Zhongguo xu mu shou yi xue hui), 1(4), 271–275.
[12] Zhou, C., Zhang, X., ShiYang, X., Wang, H., & Xiong, B. (2019). Tea polyphenol protects against cisplatin-induced meiotic defects in porcine oocytes. Aging, 11(13), 4706–4719.
[13] Cao, Y., Zhao, H., Wang, Z., Zhang, C., Bian, Y., Liu, X., Zhang, C., Zhang, X., & Zhao, Y. (2020). Quercetin promotes in vitro maturation of oocytes from humans and aged mice. Cell death & disease, 11(11), 965.
[14] Wu, M., Tang, W., Chen, Y., Xue, L., Dai, J., Li, Y., Zhu, X., Wu, C., Xiong, J., Zhang, J., Wu, T., Zhou, S., Chen, D., Sun, C., Yu, J., Li, H., Guo, Y., Huang, Y., Zhu, Q., Wei, S., … Wang, S. (2024). Spatiotemporal transcriptomic changes of human ovarian aging and the regulatory role of FOXP1. Nature aging, 4(4), 527–545.
[15] Jiao, Y., Wang, Y., Jiang, T., Wen, K., Cong, P., Chen, Y., & He, Z. (2022). Quercetin protects porcine oocytes from in vitro aging by reducing oxidative stress and maintaining the mitochondrial functions. Frontiers in cell and developmental biology, 10, 915898.
[16] Shah, M. Z. U. H., Shrivastva, V. K., Mir, M. A., Sheikh, W. M., Ganie, M. A., Rather, G. A., Shafi, M., Bashir, S. M., Ansari, M. A., Al-Jafary, M. A., Al-Qhtani, M. H., Homeida, A. M., & Al-Suhaimi, E. A. (2023). Effect of quercetin on steroidogenesis and folliculogenesis in ovary of mice with experimentally-induced polycystic ovarian syndrome. Frontiers in endocrinology, 14, 1153289.
[17] Barbe, A., Ramé, C., Mellouk, N., Estienne, A., Bongrani, A., Brossaud, A., Riva, A., Guérif, F., Froment, P., & Dupont, J. (2019). Effects of Grape Seed Extract and Proanthocyanidin B2 on In Vitro Proliferation, Viability, Steroidogenesis, Oxidative Stress, and Cell Signaling in Human Granulosa Cells. International journal of molecular sciences, 20(17), 4215.
[18] Luo, Y., Zhuan, Q., Li, J., Du, X., Huang, Z., Hou, Y., & Fu, X. (2020). Procyanidin B2 Improves Oocyte Maturation and Subsequent Development in Type 1 Diabetic Mice by Promoting Mitochondrial Function. Reproductive sciences (Thousand Oaks, Calif.), 27(12), 2211–2222.
[19] Zhou, Y., Lan, H., Dong, Z., Cao, W., Zeng, Z., & Song, J. L. (2021). Dietary proanthocyanidins alleviated ovarian fibrosis in letrozole-induced polycystic ovary syndrome in rats. Journal of food biochemistry, 45(5), e13723.
[20] Zhou, S., Zhao, A., Wu, Y., Mi, Y., & Zhang, C. (2022). Protective Effect of Grape Seed Proanthocyanidins on Oxidative Damage of Chicken Follicular Granulosa Cells by Inhibiting FoxO1-Mediated Autophagy. Frontiers in cell and developmental biology, 10, 762228.
[21] Azami, S. H., Nazarian, H., Abdollahifar, M. A., Eini, F., Farsani, M. A., & Novin, M. G. (2020). The antioxidant curcumin postpones ovarian aging in young and middle-aged mice. Reproduction, fertility, and development, 32(3), 292–303.
[22] Yin, Y. J., Zhang, Y. H., Wang, Y., Jiang, H., Zhang, J. B., Liang, S., & Yuan, B. (2023). Ferulic acid ameliorates the quality of in vitro-aged bovine oocytes by suppressing oxidative stress and apoptosis. Aging, 15(21), 12497–12512.
[23] Qian, F., Zhu, Z., Luo, C., Qi, R., Wei, L., Bo, L., Jiang, W., & Mao, C. (2025). Chlorogenic Acid Ameliorates Chronic Unpredictable Stress-Induced Diminished Ovarian Reserve Through Ovarian Renin-Angiotensin System. Molecular nutrition & food research, 69(5), e202400814.
[24] Chu, Y., Zhao, J., Zhao, Y., Li, Z., Yang, S., Chen, N., Liu, Y., Zhang, J., Zhou, L., & Chen, X. (2025). Multi-Omics Reveal the Effects and Regulatory Mechanism of Dietary Magnolol Supplementation on Production Performance of Post-Peak Laying Hens. Journal of agricultural and food chemistry, 73(7), 4027–4041.
[25] Chen, F., Zhang, H., Du, E., Jin, F., Zheng, C., Fan, Q., Zhao, N., Guo, W., Zhang, W., Huang, S., & Wei, J. (2021). Effects of magnolol on egg production, egg quality, antioxidant capacity, and intestinal health of laying hens in the late phase of the laying cycle. Poultry science, 100(2), 835–843.
[26] Gao, W., Jin, Y., Hao, J., Huang, S., Wang, D., Quan, F., Ren, W., Zhang, J., Zhang, M., & Yu, X. (2021). Procyanidin B1 promotes in vitro maturation of pig oocytes by reducing oxidative stress. Molecular reproduction and development, 88(1), 55–66.
[27] Moreira-Pinto, B., Costa, L., Felgueira, E., Fonseca, B. M., & Rebelo, I. (2021). Low Doses of Resveratrol Protect Human Granulosa Cells from Induced-Oxidative Stress. Antioxidants (Basel, Switzerland), 10(4), 561.
[28] Liang, Y., Xu, M. L., Gao, X., Wang, Y., Zhang, L. N., Li, Y. C., & Guo, Q. (2023). Resveratrol improves ovarian state by inhibiting apoptosis of granulosa cells. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology, 39(1), 2181652.
[29] Huang, Z., Pang, Y., Hao, H., Du, W., Zhao, X., & Zhu, H. (2018). Effects of epigallocatechin-3-gallate on bovine oocytes matured in vitro. Asian-Australasian journal of animal sciences, 31(9), 1420–1430.
[30] Li, N., & Liu, L. (2018). Mechanism of resveratrol in improving ovarian function in a rat model of premature ovarian insufficiency. The journal of obstetrics and gynaecology research, 44(8), 1431–1438.
[31] Yan, Z., Dai, Y., Fu, H., Zheng, Y., Bao, D., Yin, Y., Chen, Q., Nie, X., Hao, Q., Hou, D., & Cui, Y. (2018). Curcumin exerts a protective effect against premature ovarian failure in mice. Journal of molecular endocrinology, 60(3), 261–271.
[32] Barberino, R. S., Santos, J. M. S., Lins, T. L. B. G., Menezes, V. G., Monte, A. P. O., Gouveia, B. B., Palheta, R. C., Jr, & Matos, M. H. T. (2020). Epigallocatechin-3-gallate (EGCG) reduces apoptosis of preantral follicles through the phosphatidylinositol-3-kinase/protein kinase B (PI3K/AKT) signaling pathway after in vitro culture of sheep ovarian tissue. Theriogenology, 155, 25–32.
[33] Heshmati, J., Moini, A., Sepidarkish, M., Morvaridzadeh, M., Salehi, M., Palmowski, A., Mojtahedi, M. F., & Shidfar, F. (2021). Effects of curcumin supplementation on blood glucose, insulin resistance and androgens in patients with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Phytomedicine : international journal of phytotherapy and phytopharmacology, 80, 153395.
[34] Ardehjani, N. A., Agha-Hosseini, M., Nashtaei, M. S., Khodarahmian, M., Shabani, M., Jabarpour, M., Fereidouni, F., Rastegar, T., & Amidi, F. (2024). Resveratrol ameliorates mitochondrial biogenesis and reproductive outcomes in women with polycystic ovary syndrome undergoing assisted reproduction: a randomized, triple-blind, placebo-controlled clinical trial. Journal of ovarian research, 17(1), 143.
[35] Ardehjani, N. A., Agha-Hosseini, M., Nashtaei, M. S., Khodarahmian, M., Shabani, M., Jabarpour, M., Fereidouni, F., Rastegar, T., & Amidi, F. (2024). Resveratrol ameliorates mitochondrial biogenesis and reproductive outcomes in women with polycystic ovary syndrome undergoing assisted reproduction: a randomized, triple-blind, placebo-controlled clinical trial. Journal of ovarian research, 17(1), 143.
[36] Vaez, S., Parivr, K., Amidi, F., Rudbari, N. H., Moini, A., & Amini, N. (2023). Quercetin and polycystic ovary syndrome; inflammation, hormonal parameters and pregnancy outcome: A randomized clinical trial. American journal of reproductive immunology (New York, N.Y. : 1989), 89(3), e13644.
[37] Wu, C., Chen, D., Stout, M. B., Wu, M., & Wang, S. (2025). Hallmarks of ovarian aging. Trends in endocrinology and metabolism: TEM, 36(5), 418–439.
[38] Hu, H., Zhang, J., Xin, X., Jin, Y., Zhu, Y., Zhang, H., Fan, R., Ye, Y., & Li, D. (2024). Efficacy of natural products on premature ovarian failure: a systematic review and meta-analysis of preclinical studies. Journal of ovarian research, 17(1), 46.
[39] Ali Fadlalmola, H., Elhusein, A. M., Al-Sayaghi, K. M., Albadrani, M. S., Swamy, D. V., Mamanao, D. M., El-Amin, E. I., Ibrahim, S. E., & Abbas, S. M. (2023). Efficacy of resveratrol in women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized clinical trials. The Pan African medical journal, 44, 134.
[40] Malik, S., Saeed, S., Saleem, A., Khan, M. I., Khan, A., & Akhtar, M. F. (2024). Alternative treatment of polycystic ovary syndrome: pre-clinical and clinical basis for using plant-based drugs. Frontiers in endocrinology, 14, 1294406.
[41] Xu, L., Zhang, Q., Dou, X., Wang, Y., Wang, J., Zhou, Y., Liu, X., & Li, J. (2022). Fecal microbiota transplantation from young donor mice improves ovarian function in aged mice. Journal of genetics and genomics = Yi chuan xue bao, 49(11), 1042–1052.
[42] Zhang, T., Bai, S., Ding, X., Zeng, Q., Xuan, Y., Xu, S., Mao, X., Peng, H., Zhang, K., & Wang, J. (2024). Pu-erh tea theabrownin improves the ovarian function and gut microbiota in laying hens. Poultry science, 103(7), 103795.
View the article at lifespan.io








































