A recent study investigated the impact of two amino acids, phenylalanine and tyrosine, on lifespan using UK Biobank data. The researchers reported an association between tyrosine and shorter lifespan, with sex-specific differences. The results for phenylalanine were more inconsistent [1].
One by one
Model animal research has shown that protein restriction can extend lifespan. However, proteins are complex molecules built from 20 different amino acids, and to determine whether it is one or more amino acids that impact healthspan and lifespan, researchers need to investigate them individually.
For example, we recently reported that dietary methionine restriction improves healthspan and that dietary isoleucine restriction boosts lifespan in mice. On the other hand, increased protein intake, particularly leucine, can exacerbate atherosclerosis, an age-related disease. Also, some amino acids might be needed in greater amounts to help protect against aging-associated diseases. For example, we have reported that increasing glutathione levels with GlyNAC, a supplement that combines glycine and cysteine, significantly reverses age-related cognitive decline in naturally aged mice. Those reports point to the complex role of amino acids in aging and age-related diseases along with the need to investigate their individual impacts on healthspan and lifespan.
In this study, the researchers focused specifically on two amino acids, phenylalanine and tyrosine, and their impacts on lifespan, including sex-specific differences.
Tyrosine is an essential metabolite in many metabolic processes and a precursor to neurotransmitters such as dopamine, norepinephrine, and epinephrine, which regulate mood, cognition, and stress responses [2]. Animal experiments suggest that tyrosine restriction plays a role in lifespan extension, potentially by suppressing insulin signaling and the mTORC1 pathway [3].
Phenylalanine is the precursor of tyrosine. Its elevated levels were linked to such detrimental processes as telomere loss [4], inflammatory disease [5], and type 2 diabetes [6]. Its toxic derivative has been shown to shorten the lifespan of model organisms [7].
For the investigation, the researchers used a UK Biobank cohort of 272,475 participants that had all the necessary information for this analysis. However, they note some limitations of the dataset. For example, the available data included only a single measurement of tyrosine and phenylalanine levels. Therefore, they were unable to test the impacts of changes in these amino acid levels over time.
They also note that the observational design of UK Biobank data is susceptible to confounding factors arising from differences in participants’ health and socioeconomic status, the latter being especially difficult to measure accurately. To address that, they also used Mendelian randomization (MR), which uses “genetic variants as instruments, which are less affected by socioeconomic positions.” However, using these different analyses might yield different results that need to be interpreted in light of the data used in each test.
The kind of amino acid makes a difference
The initial analysis of UK Biobank data, adjusted for multiple confounders, showed an association between plasma phenylalanine and elevated all-cause mortality in the whole population and in men and women when analyzed separately. For tyrosine, the researchers also observed an association between plasma tyrosine and a higher risk of all-cause mortality in the whole study population and in men but not in women.
When specific causes of death were investigated, the analysis revealed positive associations between phenylalanine, but not tyrosine, and both cardiovascular disease (CVD) and cancer mortality, suggesting a role for phenylalanine in molecular pathways related to cardiovascular health and carcinogenesis.
Further analysis investigating the relationship between the two amino acids showed a link between a higher tyrosine-to-phenylalanine ratio and a lower overall risk of all-cause mortality in the general population and in women but not in men.
Using genetic data
A genetic analysis identified gene variants that are significantly linked to phenylalanine and tyrosine. The researchers narrowed their list to several single-nucleotide polymorphisms (SNPs, genetic variants with a single DNA base difference) and used them as instruments in their analysis. Those SNPs were “located within genes critical for amino acid metabolism, transport, and regulation.”
Then, they used those SNPs in an MR analysis to estimate their effects on lifespan. “Genetically predicted higher phenylalanine was related to longer lifespan in men but not related to lifespan in overall analysis or in women.” Those associations were the same even when different analytics methods were used.
“Genetically mimicked higher tyrosine levels were linked to a shorter lifespan in the overall population and in both sexes” in two types of analysis, and there was the same trend in the same way in the other analysis. They also conducted a similar analysis using data from different populations outside the UK, obtaining comparable results.
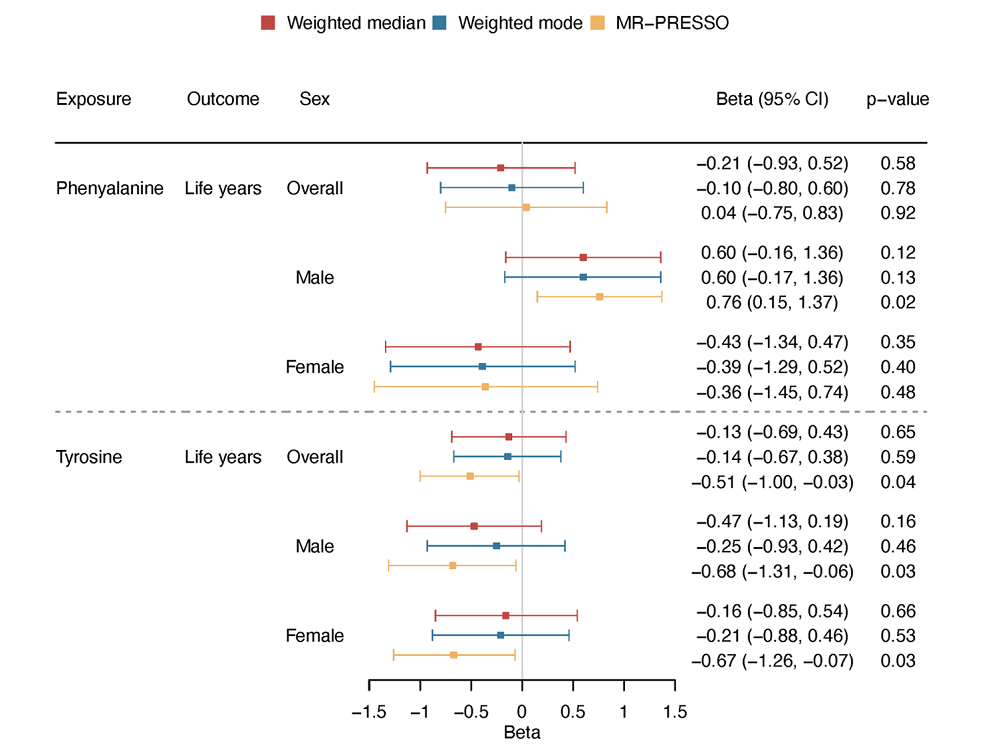
Following an MR analysis that included both amino acids, the researchers found that phenylalanine didn’t affect lifespan when controlling for tyrosine. However, the effect of tyrosine, associated with shorter lifespan in men but not women persisted even after controlling for phenylalanine.
Tyrosine reduction as a possible intervention
Summarizing, the researchers observed an association between tyrosine and shorter lifespan in observational and MR studies, which was independent of phenylalanine. The effect was stronger in men than in women, possibly due to sex-specific differences or insufficient statistical power in some tests to detect it. Future studies with larger sample sizes might confirm or debunk these results.
On the other hand, the phenylalanine results were less consistent. An observational analysis found an association between plasma phenylalanine and elevated all-cause mortality, but in an MR analysis, in which the researchers controlled for tyrosine, phenylalanine did not affect lifespan.
The researchers suggest that, based on their results and on rodent studies that also implicate tyrosine in lifespan, “reducing tyrosine in people with elevated concentrations may contribute to prolonging lifespan”. However, there is a need for a better understanding of the molecular mechanisms that link tyrosine restriction to lifespan extension.
Literature
[1] Zhao, J. V., Sun, Y., Zhang, J., & Ye, K. (2025). The role of phenylalanine and tyrosine in longevity: a cohort and Mendelian randomization study. Aging, 17(10), 2500–2533.
[2] Fernstrom, J. D., & Fernstrom, M. H. (2007). Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. The Journal of nutrition, 137(6 Suppl 1), 1539S–1548S.
[3] Kosakamoto, H., Sakuma, C., Okada, R., Miura, M., & Obata, F. (2024). Context-dependent impact of the dietary non-essential amino acid tyrosine on Drosophila physiology and longevity. Science advances, 10(35), eadn7167.
[4] Eriksson, J. G., Guzzardi, M. A., Iozzo, P., Kajantie, E., Kautiainen, H., & Salonen, M. K. (2017). Higher serum phenylalanine concentration is associated with more rapid telomere shortening in men. The American journal of clinical nutrition, 105(1), 144–150.
[5] Neurauter, G., Schröcksnadel, K., Scholl-Bürgi, S., Sperner-Unterweger, B., Schubert, C., Ledochowski, M., & Fuchs, D. (2008). Chronic immune stimulation correlates with reduced phenylalanine turnover. Current drug metabolism, 9(7), 622–627.
[6] Guasch-Ferré, M., Hruby, A., Toledo, E., Clish, C. B., Martínez-González, M. A., Salas-Salvadó, J., & Hu, F. B. (2016). Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes care, 39(5), 833–846.
[7] Dato, S., Hoxha, E., Crocco, P., Iannone, F., Passarino, G., & Rose, G. (2019). Amino acids and amino acid sensing: implication for aging and diseases. Biogerontology, 20(1), 17–31.
View the article at lifespan.io








































