In a new study, extracellular vesicles (EVs) derived from mesenchymal stem cells (MSCs) improved spatial working memory in rhesus macaques, suggesting a possible reversal of age-related cognitive decline [1].
The “normal” decline
Even “healthy” aging (not accompanied by obvious age-related diseases such as dementia) leads to cognitive impairments, particularly in working memory, executive function, and recognition memory [2]. This incessant cognitive decline, which starts at midlife, is linked to myelin pathology in the brain, rather than widespread neuronal loss [3].
In this new study published in the journal GeroScience, researchers at Boston University Chobanian & Avedisian School of Medicine tested the hypothesis that extracellular vesicles derived from young MSCs can reverse this age-related decline in rhesus monkeys.
EVs are tiny lipid bubbles secreted by cells. Containing molecules such as proteins and RNA, they are used for intercellular communication. EVs derived from stem cells have been shown to recapitulate some of the benefits of stem cell therapies without the immunogenic risk that comes with them [4].
Thirteen late middle-aged rhesus monkeys (17-24 years old, roughly equivalent to 51-72 human years) were selected for this study. The animals underwent baseline cognitive testing and MRI scans before being randomly assigned to MSC-EV or control groups for treatment. Bone-marrow MSC-derived EVs were taken from a single young monkey (about 6 years old) and administered intravenously bi-weekly for 18 months. The control group got the same schedule of sham injections without EVs.
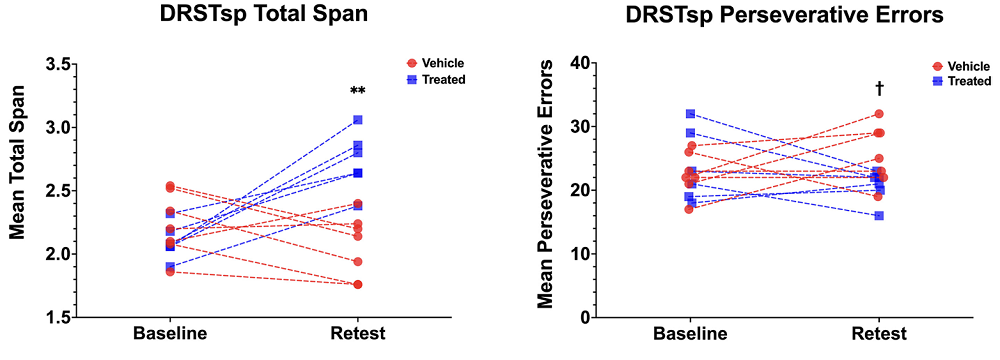
MRI scans were conducted at baseline and every six months during treatment to monitor changes in white matter integrity and functional connectivity. For cognitive assessments, the team used two tests: the Delayed Non-Matching to Sample (DNMS) task and the Delayed Recognition Span Task – Spatial (DRSTsp). The former evaluates recognition memory, while the latter assesses spatial working memory.
Improvements in working memory and white matter
Over the course of the study, MSC-EV treatment led to significant improvements in spatial working memory. The treatment not only maintained performance but also reversed age-related cognitive decline, with treated monkeys performing at levels comparable to younger monkeys in one of the team’s previous studies.
It was, however, a bit more complicated with recognition memory. While the difference between the two groups at the end of the study was not statistically significant, the control group had better baseline scores. When this and some other variables were accounted for, the results of one part of the DNMS test (the two-minute delay) did cross the significance threshold.
MRI results indicated improvements in white matter structural integrity in regions such as the right middle temporal area and the fornix along with preserved functional connectivity in the MSC-EV group compared to the control group. In correlation analyses, certain connectivity patterns at the end of treatment were associated with better performance, suggesting that structural and functional brain changes are associated with cognitive outcomes.
While this study did not include biochemical or histological readouts, the same group has previously shown that MSC-derived EVs reduce microglia-mediated neuroinflammation and injury-related pathology and support synapse remodeling in aged rhesus monkeys after cortical injury. In related rodent and primate models, similar EV therapies have been shown to also promote remyelination and reduce white-matter damage.
Outline for future studies
“By applying secreted stem cells, specifically EVs, we found that the aging brain retains a remarkable capacity for resilience. Our findings suggest that aging is not set in stone; that brain health can be supported and maintained even in older age,” explained corresponding author Evan Mackie, a Ph.D. student in the school’s department of anatomy and neurobiology.
“Because similar vulnerabilities in brain structure and function also occur in conditions such as Alzheimer’s disease, multiple sclerosis, stroke and brain injury, this approach may one day help protect the brain in both healthy aging and disease,” added senior author Tara L. Moore, Ph.D., professor of anatomy and neurobiology.
This study had several limitations. For instance, the MSC-EV group was not sex-balanced (four females, two males). Given that the females showed better baseline scores in some of the tasks, this might have affected the results.
The researchers recommend larger sample sizes and longer treatment periods for future studies to enhance statistical power and explore treatment effects. If those future studies replicate the results of this one, investigation into molecular changes and histological analysis of brain pathways is still needed to better understand the mechanisms behind the cognitive improvements.
Literature
[1] Mackie, E. C., Cheng, C. H., Alibrio, M. N., Rutledge, C., Xin, H., Chopp, M., … & Moore, T. L. (2025). Mesenchymal cell-derived extracellular vesicles ameliorate age-related deficits in working memory and in vivo MRI measures of white matter structure and function in rhesus monkeys. GeroScience, 1-25.
[2] Buckner, R. L. (2004). Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron, 44(1), 195-208.
[3] Gong, Z., Bilgel, M., Kiely, M., Triebswetter, C., Ferrucci, L., Resnick, S. M., … & Bouhrara, M. (2023). Lower myelin content is associated with more rapid cognitive decline among cognitively unimpaired individuals. Alzheimer’s & Dementia, 19(7), 3098-3107.
[4] Tan, F., Li, X., Wang, Z., Li, J., Shahzad, K., & Zheng, J. (2024). Clinical applications of stem cell-derived exosomes. Signal transduction and targeted therapy, 9(1), 17.
View the article at lifespan.io








































