In Aging, researchers have described how they removed visceral fat from older male mice by targeting the metabolic regulatory protein CD47.
A key regulator of metabolism
Visible fat carried around the body is subcetaneous fat. While this kind of fat is obviously not healthy to have in large amounts, it is not as immediately dangerous as visceral fat, which accumulates around organs and drives many metabolic diseases [1]. This metabolic damage is often associated with similar age-related problems [2], including sarcopenia, a loss of muscle mass that leads to frailty [3].
To combat visceral fat, the researchers focus on CD47, a multifunctional membrane protein that increases during aging and is known to drive several age-related disorders, including a loss of the ability to create new blood vessels [4]. Cancers also use it to protect themselves from the immune system [5].
Most relevant to this research, however, is the fact that excessive CD47 has been repeatedly found to lead to age-related metabolic disorders in animal models, including obesity and diabetes [6]. These researchers previously found that a CD47 deficiency leads to the browning of white fat, putting it into a state in which it can be burned for heat, and then encourages that burning [7]. Curiously, further work found that these results only appear to apply to male animals [8].
A potential treatment for fat generation
This work takes that previous research a bit further; while the previous work used modified mice, this uses an actual treatment: an antisense oligonucleotide that specifically targets CD47 (CD47 ASO). A group of 20-month-old wild-type Black 6 mice was injected twice a week, along with a saline-injected group and a control ASO group.
Overall body weight did not significantly change between the groups. Total fat mass, on the other hand, was significantly decreased in the CD47 ASO group compared to either of the controls, and this was accompanied by a significant loss of visceral fat.
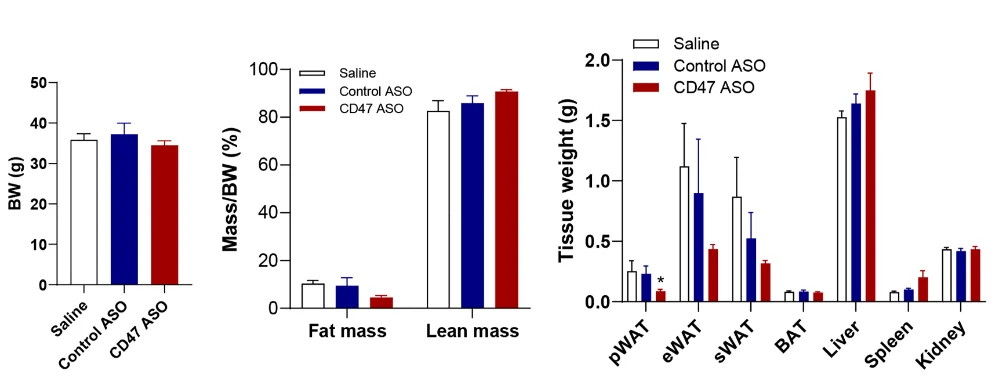
There was also a marked improvement in diabetes-related biomarkers; the treated mice were better able to handle glucose, and HOMA-IR, a biomarker of insulin sensitivity, was improved. The fat cells themselves had become smaller with this treatment as well.
These findings were accompanied by marked changes in gene expression. While many genes related to fat usage were unchanged, genes related to the formation of fat were significantly downregulated in the CD47 ASO group. Additionally, there appeared to be upregulation of genes related to the anti-inflammatory M2 macrophage type, although most genes related to the pro-inflammatory M1 type were unaffected.
The lack of CD47 discouraging fat cells from forming was confirmed in a cellular study. The researchers grew and differentiated fat cells for 15 days, subjecting some of them to CD47 ASO. The cells so treated were not significantly affected for the first week, but by the end of this experiment, at which point the cells had become senescent due to replication, lipogenesis-related genes were significantly downregulated. A closer examination revealed a more nuanced finding: this approach appears to encourage cells to differentiate into fat cells while preventing senescent cells from accumulating fat.
The researchers noted the very specific effects of this treatment: while there was a slight improvement in the liver’s ability to metabolize glucose, CD47 ASO had no apparent effects on skeletal muscle and other tissues. These effects were sex-specific in mice, and it is not yet clear if they apply to human beings; a clinical trial would need to be done to determine if targeting CD47 could be effective in helping elderly men or women to lose weight.
Literature
[1] Shuster, A., Patlas, M., Pinthus, J. H., & Mourtzakis, M. (2012). The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. The British journal of radiology, 85(1009), 1-10.
[2] Tam, B. T., Morais, J. A., & Santosa, S. (2020). Obesity and ageing: Two sides of the same coin. Obesity Reviews, 21(4), e12991.
[3] Nishikawa, H., Asai, A., Fukunishi, S., Nishiguchi, S., & Higuchi, K. (2021). Metabolic syndrome and sarcopenia. Nutrients, 13(10), 3519.
[4] Ghimire, K., Li, Y., Chiba, T., Julovi, S. M., Li, J., Ross, M. A., … & Rogers, N. M. (2020). CD47 promotes age-associated deterioration in angiogenesis, blood flow and glucose homeostasis. Cells, 9(7), 1695.
[5] Sun, J., Chen, Y., Lubben, B., Adebayo, O., Muz, B., & Azab, A. K. (2021). CD47-targeting antibodies as a novel therapeutic strategy in hematologic malignancies. Leukemia Research Reports, 16, 100268.
[6] Maimaitiyiming, H., Norman, H., Zhou, Q., & Wang, S. (2015). CD47 deficiency protects mice from diet-induced obesity and improves whole body glucose tolerance and insulin sensitivity. Scientific reports, 5(1), 8846.
[7] Li, D., Gwag, T., & Wang, S. (2021). Absence of CD47 maintains brown fat thermogenic capacity and protects mice from aging-related obesity and metabolic disorder. Biochemical and biophysical research communications, 575, 14-19.
[8] Li, D., Gwag, T., & Wang, S. (2023). Sex differences in the effects of brown adipocyte CD47 deficiency on age-related weight change and glucose homeostasis. Biochemical and biophysical research communications, 676, 78-83.
View the article at lifespan.io








































