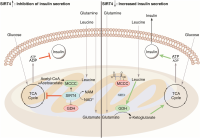
This also raises the question of whether a ketogenic diet is likely to result in mitochondrial fusion, and the consequent boost to ATP production and lipogenesis (fat storing). It seems obvious now that this will not be the case, and that the opposite is likely - keto is likely to be similar to starvation in that low insulin levels will suppress mTOR and raise SIRT4 expression levels (apart from around meal times). Similarly OPA1 is likely to be repressed and mitochondria fission and mitophagy increased, and this jives with what we know of a ketogenic diet protecting from cancer and aging.
This is different to the case of statins, where lipogenesis is blocked, but OPA1 is not - in fact it has been shown that this is raised (see https://www.ncbi.nlm...bmed/27720611),which leads to mitochondrial fusion. And this potentially explains how statins can lead to de-differentiation (via GTPases, and also mitochondrial fusion - same as Stearic acid) and maybe longer telomeres (higher SIRT4 through interaction with OPA1, though it's not clear how these then get located in the nucleus). However the downregulated mitophagy would eventually lead to dysfunctional mitochondria (as is widely reported by long term users).
Stearic acid upregulates mitochondrial fusion via the strong signal of fatty acids ready to be metabolised.
Looks like short life statins like pravastatin would be the best.
How would statins work combined with a ketogenic diet?
P.S. I haven't found studies where insulin regulates mTor, looks like that link work in the opposite direction - mTor inhibition downregulates insulin secretion
Edited by Andey, 04 March 2019 - 02:45 PM.