.
F U L L T E X T S O U R C E : International Journal of Molecular Sciences
Abstract
A balanced metabolic profile is essential for normal human physiological activities. Disproportions in nutrition give rise to imbalances in metabolism that are associated with aberrant immune function and an elevated risk for inflammatory-associated disorders. Inflammation is a complex process, and numerous mediators affect inflammation-mediated disorders. The available clinical modalities do not effectively address the underlying diseases but rather relieve the symptoms. Therefore, novel targeted agents have the potential to normalize the metabolic system and, thus, provide meaningful therapy to the underlying disorder. In this connection, polyphenols, the well-known and extensively studied phytochemical moieties, were evaluated for their effective role in the restoration of metabolism via various mechanistic signaling pathways. The various flavonoids that we observed in this comprehensive review interfere with the metabolic events that induce inflammation. The mechanisms via which the polyphenols, in particular flavonoids, act provide a promising treatment option for inflammatory disorders. However, detailed clinical studies of such molecules are required to decide their clinical fate.
1. Introduction
Multicellular organisms fight infections, manage different external and internal damages, and maintain the body’s energy balance, particularly under energy deficit conditions. In overall summation, the immune system and metabolic pathways are among the primary essentialities, without which the animal kingdom would cease to exist [1]. Furthermore, both the immune and metabolic pathways co-evolved in a manner in which they are closely linked and interdependent. Nutritional imbalances disrupt metabolism, leading to irregular immune function and an elevated risk for inflammatory-associated disorders [2]. In ancient history, inflammation was characterized based on the visual observations of five cardinal signs specifically named as rubor (redness), tumor (swelling), calor (heat), dolor (pain), and lastly function laesa (loss of function) [3]. In short, inflammation could be described as a response of living tissue to local injury [4,5]. There is evidence to support that inflammation plays a decisive role in neoplastic progression. This concept is based on the relationship between incessant inflammatory activities due to viruses, bacteria, parasites, infections, and carcinogenesis and their effects within the organs and tissues [6,7,8,9,10].
Inflammation can be further divided into acute and chronic. The local effects of acute inflammation include modifications of metabolic and functional activities of polymorphonuclear cells and macrophages. The systemic effects of acute inflammation include modification of immune response and non-specific defenses against infection and neoplasia [11]. Acute inflammation can also lead to fever and leukocytosis [4]. Chronic inflammation is characterized by the medical condition of chronic inflammatory disease. This medical condition can be defined as a lengthy and persistent pro-inflammatory state marked specifically by the formation of new connective tissue [12]. There are many diseases included in this category: autoimmune disease, metabolic syndromes, neurodegenerative disease, chronic inflammatory bowel disease, chronic obstructive pulmonary disease, and cardiovascular disease (CVD) [12,13]. Among the abovementioned diseases and syndromes, metabolic syndromes are strongly associated with chronic inflammation [1]. Inflammatory reaction pathways involve various receptors and molecules, such as Toll-like receptors (TLRs) or nucleotide oligomerization domain (NOD)-like receptors (NLRs). These receptors activate major mitogen-activated protein kinase (MAPK) cascades and stimulate translocation of regulatory nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [7]. It was documented that the expression of pro-inflammatory cytokine mediators orchestrates an indispensable role in various CVDs, metabolic syndromes, and atherosclerosis [14,15,16,17,18].
A number of investigators reported the natural amelioration of inflammation via the use of polyphenols due to their anti-inflammatory activities [19,20,21]. It was found that numerous polyphenols, such as flavonoids, are active suppressors of inflammatory cytokines, modulators of transcription factors and inflammation-related pathways, and reducers of accumulated of nitric oxide (NO) or reactive oxygen species (ROS) [22]. For example, a species-specific flavonoid, glabridin, was found to attenuate mediators of inflammation including nitric oxide (NO), tumor necrosis factor-α (TNF-α), and interlukin (IL)-1β in THP-1 cells, RAW 264.7 cells, and J774a.1 cells [23,24,25,26]. Another investigator showed that glabridin also inhibited the maturation of dendritic cells by blocking NF-κB and MAPK signaling cascade [27]. Similarly, phloretin, another important polyphenol, was found to inhibit expression of IL-8, C–X–C motif chemokine 10, and TNF-α messenger RNAs (mRNAs) [28].
Oxidative stress and inflammatory mediators are well known for their role in generating ROS and reactive nitrogen species (RNS) by affecting NADPH oxidase and nitric oxide synthase (NOS) respectively. ROS trigger redox-sensitive kinases, such as apoptosis signal-regulating kinase 1 (ASK1), which in turn activates downstream MAPKs, NF-κB, and activator protein 1 (AP-1), resulting in the induction of inflammatory gene expression. Research shows that phenolic phytochemicals possess strong antioxidant activity due to the presence of hydroxyl groups within their aromatic rings [29,30]. The molecular mechanism involved lies in the capacity of the phenolic phytochemicals to increase the level of anti-inflammatory genes, such as glutathione peroxidase (GPx), superoxide dismutase (SOD), and hemeoxygenase-1 (HO-1), via activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) [30].
To manage metabolic syndromes, global strategies initially focused on lifestyle changes such as diet and physical activity. Diets rich in plant-derived products with a high content of bioactive compounds, mono/polyunsaturated fatty acids, and polyphenols were shown to lower related risks of metabolic syndrome [31,32,33]. Polyphenols are available in various regular diets, indicating negligible side effects if taken in a controlled manner. This fact makes them a good candidate for their use in various clinical trials in treatment of various diseases. There is a large body of evidence to suggest that polyphenols play an effective role as anti-inflammatory agents in various metabolic diseases [34,35,36]. However, there is no review article that specifically concentrates on this subject. In addition, there is little information on the utilization of polyphenols to ameliorate the effect of metabolic disorders, as well as their in vitro and in vivo evaluation.
The purpose of this review is to summarize recent data on dietary polyphenols, with a special emphasis on flavonoids, which affect the inflammation involved in various metabolic disorders, and to gather the results of clinical studies on the chronic supplementation of flavonoids on metabolic syndromes features.
2. Inflammation and Metabolic Disorders
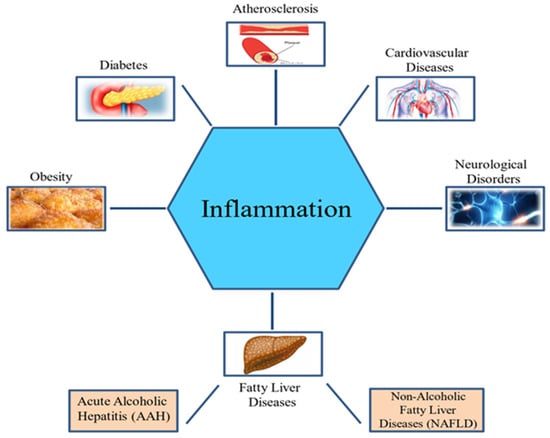
Inflammation is a defensive strategy developed in higher organisms in reaction to the harmful effects of tissue injury, microbial infection, and other detrimental conditions. It is an important immune response by the host that aids in tissue repair and the elimination of harmful stimuli [37]. However, long-term inflammation is often harmful and can cause several metabolic diseases [13] (Figure 1).
Figure 1. Inflammation and metabolic disorders.
Moreover, high caloric intake coupled with inactive lifestyle leads to increased incidence of obesity, type 2 diabetes, and cardiovascular diseases, which are associated with chronic diseases [38,39,40]. Metabolism and inflammation are interrelated. Metabolic disorders display a strong inflammatory foundation, and inflammation is also linked to metabolic changes. The complex interaction between inflammatory and metabolic pathways is underscored by the biological and functional similarity of macrophages and adipocytes. In both cell types, similar gene expression is observed. Several adipocyte proteins are expressed by macrophages such as peroxisome proliferator-activated receptor-γ (PPARγ), adiponectin protein 2 (aP2), and adipocyte/macrophage fatty-acid binding protein (FABP). In addition, adipocytes express many “macrophage” gene products, such as IL-6, TNF-α, and matrix metalloproteinases (MMPs) [41,42].
Monocytes at infection sites differentiate into macrophages under the influence of the local cytokine environment [43]. Based on function and cytokine expression, differentiated macrophages are classified as M1 and M2 macrophages. M1 macrophages produce pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α, which result in the mobilization of neutrophils and help to initiate the innate immune response against pathogens. Furthermore, T cells are stimulated by the cumulative action of pro-inflammatory cytokines and their ligands. M1 macrophages and T lymphocytes are linked to chronic inflammation in metabolic disorders. M2 macrophages are anti-inflammatory mediators that participate in wound healing and the angiogenic process [44].
The transcription factor NF-κB is a well-known inflammatory and immunological mediator that connects inflammation to metabolic responses. It helps to better understand metabolic diseases and provides insight into the development of therapeutic strategies. Pathogen-associated molecular patterns (PAMPs) and cytokines trigger cell surface receptors, including TLRs, which initiate a signaling pathway that activates NF-κB [45]. NF-κB-regulated gene expression is responsible for the differentiation of various types of immune cells. Involvement of NF-κB was revealed in three common metabolic disorders: atherosclerosis, obesity, and insulin resistance. Extracellular stimuli control the activity of NF-κB. The nuclear localization and transcriptional function of NF-κB is prevented by inhibitor of κB (IκB). IkB sequesters NF-κB in the cytoplasm of resting cells. Interaction of cytokine or PAMP with cell surface receptors begins a signaling sequence that activates the inhibitor of κB kinase (IKK) complex. Phosphorylation of IκB by IKK stimulates its break down that releases NF-κB and, consequently, NF-κB moves inside the nucleus and promotes transcription of target genes. Research findings suggest that the IKKγ/IKKβ complex initiates NF-κB-regulated gene expression downstream of TLRs and cytokine receptors [45].
Most resting cells do not express the IKK3, but NF-κB induces its transcription downstream of inflammatory stimuli [46]. Research findings showed the involvement of the non-canonical IKK kinase IKK3 in high-fat diet-induced obesity. In response to excess nutrients, IKK3 expression increases approximately 40 times in fat cells and fat-infiltrating macrophages [47].
Deficiency of IKK3 causes uncoupling of obesity from a fat-rich diet by increasing energy consumption, thermogenesis, and aerobic respiration. IKK3 knockdown mice are protected from pro-inflammatory pathway activation, chronic inflammation in liver and adipose tissue, and diet-induced insulin resistance because of lesser weight gain and possibly improper cytokine production and signaling pathway [48,49,50,51,52]. Hence, IKK family signaling cascade performs a vital function in overnutrition-induced obesity, as well as in metabolic disease.
Inflammatory processes are recognized to induce atherosclerosis. Atherosclerosis begins with the accumulation of excess unmetabolized lipoproteins in the blood. Oxidized lipoproteins induce vascular endothelia to release chemokines, macrophage inflammatory protein-1α and monocyte chemoattractant protein-1 (MCP-1), which attract leukocytes to the inflammation site [48,49]. Association of oxidative stress and mitochondrial dysfunction with inflammation is implicated in type 2 diabetes, leading to insulin resistance in muscle and adipocyte cells, as well as impaired insulin secretion by the β cells of the islets of Langerhans [50,51]. Oxidative stress activates NF-κB and c-Jun N-terminal kinase (JNK) pathways and, thus, shows the ability of ROS to cause insulin resistance [52,53,54].
.../...
.