.
F U L L T E X T S O U R C E : Frontiers
Our understanding of the molecular basis of aging has greatly increased over the past few decades. In this review, we provide an overview of the key signaling pathways associated with aging, and whose modulation has been shown to extend lifespan in a range of model organisms. We also describe how these pathways converge onto autophagy, a catabolic process that functions to recycle dysfunctional cellular material and maintains energy homeostasis. Finally, we consider various approaches of therapeutically modulating these longevity pathways, highlighting exercise as a potent geroprotector.
Introduction
In the past two decades, the molecular signatures of aging have been started to be uncovered. A remarkable conservation of these cell signaling pathways has been shown across various invertebrate and vertebrate species (Kenyon, 2010). Autophagy is a cellular process that has emerged as a nexus at which these various pathways have been shown to converge. Autophagy is the catabolic process by which the cell eliminates unnecessary cellular components to maintain energy homeostasis and prevent the build-up of toxic material. There are three forms of autophagy—macroautophagy, microautophagy, and chaperone-mediated autophagy. In this review, we will only discuss macroautophagy (which we will henceforth refer to simply as “autophagy”). This review will provide an overview of the cell signaling pathways that are associated with longevity, and discuss how they all converge onto autophagy. We will also discuss how established anti-aging approaches including exercise, caloric restriction, and pharmaceutical therapeutics affect these pathways to regulate autophagy in ways that are geroprotective and possibly longevity-enhancing.
Evidence That Autophagy is Associated With and Necessary for Longevity
Autophagic activity has been shown to decline with age in various animal models. For example, body-wide quantification of autophagic flux in Caenorhabditis elegans revealed a general decline in activity in various tissues, including the intestine and neurons (Chang et al., 2017). A similar decline in function has been observed in mammals. For example, electron microscopy analysis of aged mouse livers revealed a depression in the rate of autophagic vesicle formation (Terman, 1995).
Various groups have identified a necessary role of autophagy in mediating the effects of longevity-enhancing mutations. The Levine group was the first to demonstrate that inhibiting autophagy in a long-lived mutant model nullifies the longevity-promoting effects of the mutation. C. elegans worms that carry a loss-of-function mutation in their daf-2 gene [which encodes for a common single insulin/Insulin-like Growth Factor (IGF)-1 Receptor in this organism] live significantly longer than their wild-type counterparts. They demonstrated that RNAi-mediated knockdown of the autophagy gene bec-1 significantly reduced the lifespan of the daf-2 mutants, clearly identifying autophagy as a process that is required for the increased longevity of this mutant (Melendez et al., 2003).
To demonstrate a causal relationship between autophagy and longevity, some groups have evaluated the effects of overexpressing autophagy genes. A positive relationship between autophagic activity and lifespan was first demonstrated in Drosophila. Neuron-specific overexpression of the Atg8a gene resulted both in an increase in lifespan and a reduction in the accumulation of toxic protein aggregates in neurons (Simonsen et al., 2008). Similarly, body-wide overexpression of Atg5 resulted in a significant increase in lifespan in mice (Pyo et al., 2013). Increase in autophagy via disruption of the beclin1-BCL2 complex has been shown to promote both healthspan and lifespan in mice (Fernandez et al., 2018).
Autophagy and the Hallmarks of Aging
Guido Kroemer and colleagues have recently published an excellent overview of the molecular underpinnings of aging, in which they enumerate the following nine hallmarks of aging—genomic instability, telomere shortening, epigenetic alterations, loss of proteostasis, dysregulated nutrient sensing, mitochondrial dysfunction, cell senescence, stem cell loss, and altered intercellular communication (Lopez-Otin et al., 2013). Remarkably, autophagy has been shown to be intimately involved in nearly all of these processes. Autophagy can mitigate the effects of genomic instability by reducing the production of DNA-damaging reactive oxygen species (ROS) production, and by promoting the recycling of DNA repair proteins (Vessoni et al., 2013). Although autophagy is unable to revert or stall telomere attrition, recent work has shown that telomere dysfunction directly stimulates autophagy to promote the death of precancerous cells (Nassour et al., 2019). While autophagy is not thought to have a direct relationship with epigenetic alterations, it has canonical roles in maintaining proteostasis (Kern and Behl, 2019), nutrient sensing (Dagon et al., 2015), and mitochondrial health via mitophagy (Palikaras et al., 2018). While the relationship between autophagy and senescence is complex and requires further disentangling, autophagy has been shown to play an essential role in the maintenance of stem cells (Boya et al., 2018). Finally, autophagy maintains proper immune function (a key component of intercellular communication) by preserving phagocytic activity and controlling levels of inflammation (Cuervo and Macian, 2014). In summary, autophagy has been shown to counter the effects of the majority of the presented hallmarks of aging.
Longevity Pathways and Autophagy
Four well-studied pathways that are known to regulate aging, and whose modulation has been shown to influence the rate of aging are Insulin/IGF-1, mechanistic target of rapamycin (mTOR), AMP-activating protein kinase (AMPK), and Sirtuin pathways (Kenyon, 2010). In this section, we will discuss the relationship between each of these pathways and longevity, their effects on autophagy, and the effects of aging and exercise on these pathways with respect to autophagy. Figure 1 illustrates how these various pathways converge onto, and activate, autophagy.
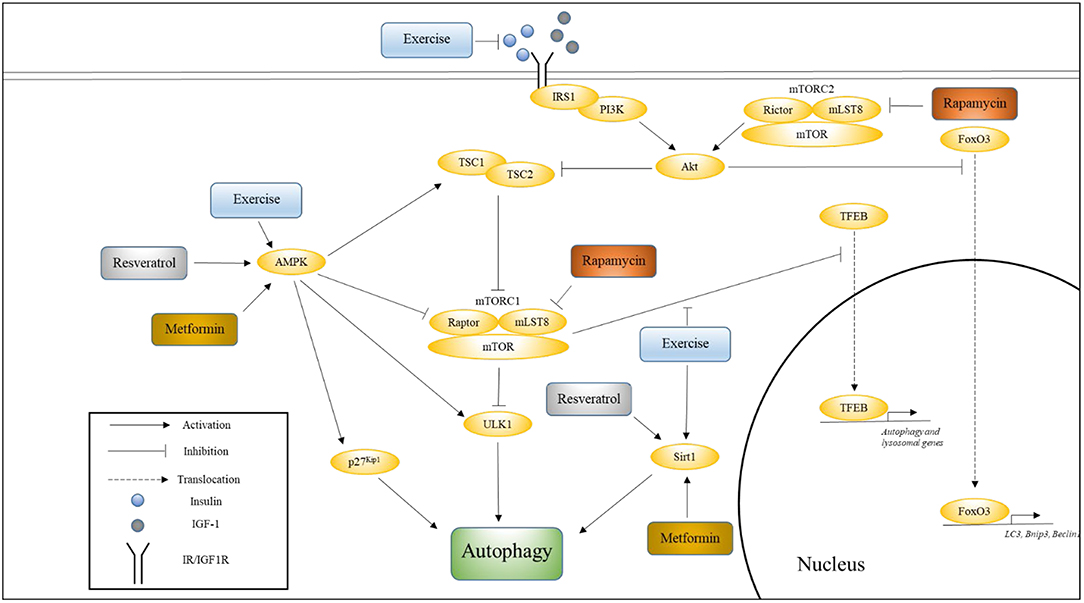
Figure 1. The influence of exercise on cell signaling pathways that regulate autophagy. This figure shows how various pathways associated with longevity converge onto autophagy, and how exercise influences these pathways. Also indicated are the nodes upon which Metformin, Rapamycin, and Resveratrol are thought to act. Please see text for details.
Insulin/IGF-1 Signaling (IIS)
IIS and Longevity
The insulin/IGF-1 (IIS) pathway was the first pathway to be shown to affect aging (Kenyon et al., 1993). C. elegans worms with a loss-of-function mutation in their daf-2 gene experienced a >2-fold extension in lifespan compared to wild-type. Inhibition of this pathway in vertebrate models has also been shown to extend lifespan, but to a lesser degree and in a more inconsistent manner. Female 129/SvPas mice heterozygous for the IGF-1 receptor null allele (Igf1r+/−) have been shown to live significantly longer (33%) than wild-type females, while male mutant mice demonstrated no such lifespan enhancing benefits (Holzenberger et al., 2003). Subsequent work has demonstrated that these benefits are strain dependent, as female C57BL/6J Igf1r+/− mice experienced a more modest (albeit significant) increase in lifespan compared to wild-type controls (Xu et al., 2014). In contrast, both male and female fat-specific insulin receptor knockout (FIRKO) mice showed a significant increase in lifespan (Bluher et al., 2003), possibly indicating that insulin signaling is more relevant to longevity than IGF-1 signaling. Alternatively, perhaps these differences between C. elegans and mice can be attributed to the fact that daf-2 encodes for a receptor that shows significant homology to both the IGF-1 receptor and the insulin receptor (Kimura et al., 1997), suggesting that dual knockout (or knockdown) of these receptors is necessary to achieve enhanced lifespan extension. In support of this supposition is the finding that both male and female mice that are null for the insulin receptor substrate protein 1 (Irs1) experienced significant extensions in lifespan (Selman et al., 2011). Irs1 is an adaptor protein that mediates the actions of both insulin and IGF-1.
Effects of IIS on Autophagy
The C. elegans daf-2 mutants exhibit a pronounced increase in autophagic activity compared to wild-type worms, indicating that the IIS pathway suppresses autophagy (Hansen et al., 2008). Indeed, activation of the IIS pathway is known to inhibit autophagy via the activation of mTORC1 and inhibition of FoxO signaling. FoxO proteins are transcription factors whose translocation to the nucleus is blocked via phosphorylation of Akt, which is a key kinase in the IIS pathway (Sandri et al., 2004). Under conditions of nutrient deprivation (and suppressed IIS), FoxO3 upregulates autophagy by promoting the expression of autophagy-related genes, including LC3, Bnip3, and Beclin1 (Mammucari et al., 2007; Zhao et al., 2007).
Effects of Age on IIS
As people age they enter a stage known as somatopause, during which they experience a decline in circulating growth hormone (GH) and IGF-1 levels (Junnila et al., 2013). Somatopause has also been detected in other mammals (Bartke, 2008). Paradoxically, centenarians have been shown to have significantly lower levels of circulating IGF-1 (Bonafe et al., 2003). Additionally, the offspring of centenarians have been shown to have both lower levels of circulating IGF-1 and lower IGF-1 activity compared to controls whose parents both died relatively young (Vitale et al., 2012). Perhaps the potential negative effects of lower GH/IGF-1 levels (e.g., lower levels of anabolism) are offset by a less pronounced decline in systemic autophagic activity. In support of this idea, healthy centenarians have been shown to have significantly higher levels of circulating beclin-1 compared to both young patients who have experienced an acute myocardial infarction and healthy young controls (Emanuele et al., 2014). This observation has been independently confirmed in a recent study that also showed a general increase in the expression of genes in the autophagy-lysosomal pathway in centenarians (Xiao et al., 2018).
Unlike IGF-1, circulating insulin levels generally increase with age. Aging is associated with hyperinsulinemia and insulin resistance that are caused by greater secretion of insulin in response to the same stimulus compared to younger individuals (Gumbiner et al., 1989). In contrast, centenarians have been shown to exhibit both a lower degree of insulin resistance and preserved β-cell function (Paolisso et al., 2001). Additionally, increased insulin sensitivity and lower mean fasting insulin levels have been observed in the offspring of nonagenarians compared to their partners (Rozing et al., 2010). A causal relationship between higher circulating insulin levels and decreased hepatic autophagy has been demonstrated in mice (Liu et al., 2009).
In summary, aging is associated with decreasing levels of autophagic activity that are partially the result of dysregulated IIS. Healthy centenarians, who do not experience the typical effects of normal aging, display both enhanced autophagy and better-preserved and regulated IIS.
Effects of Exercise on IIS
There is strong evidence to suggest that exercise promotes both healthspan and lifespan in worms (Chuang et al., 2016), flies (Piazza et al., 2009), and mammals (Cartee et al., 2016). In association, there is extensive evidence that indicates that exercise effectively suppresses insulin resistance and hyperinsulinemia (Ryan, 2000). Various population studies have shown inverse associations between physical activity and the incidence of type 2 diabetes mellitus, and both regular aerobic and resistance exercise have been recommended by the American Diabetes Association, especially for patients with type 2 diabetes (Colberg et al., 2016). Vigorous endurance exercise has been shown to decrease plasma insulin concentration and increase insulin sensitivity in subjects in their 60 s (Kirwan et al., 1993). A recent study has shown that a more gentle exercise regimen involving 20 min of resistance band exercise and 30 min of walking three times a week for 12 weeks is sufficient to improve insulin resistance in elderly women aged 70–80 years (Ha and Son, 2018). Mechanistically, one of the ways in which exercise is thought to increase insulin sensitivity is via contraction-stimulated glucose uptake, which involves the activation of AMPK. Importantly, exercise has also been shown to promote systemic autophagy (He et al., 2012). Perhaps acute exercise counteracts the autophagy-suppressing effects of IIS via the activation of autophagy promoters (such as AMPK), and regular exercise maintains long-term autophagic activity via preservation of insulin sensitivity and the consequent reduction in circulating insulin levels. Finally, the insulin sensitizing role of exercise-regulated myokines is discussed in a later section.
mTOR
mTOR and Longevity
As with the IIS pathway, inhibition of mTOR results in increased longevity. C. elegans deficient in TOR, like the previously-described daf-2 mutants, also displayed a doubling in lifespan (Vellai et al., 2003). Suppression of mTOR to ~25% of wild-type levels in mice carrying two hypomorphic mTOR alleles has also been shown to significantly extend median lifespan in both male and female mice (Wu et al., 2013). However, these mice experience lifespan extension of only ~20%, which approximately mirrors the lifespan extension seen in mice with suppressed IIS, as previously noted.
Effects of mTOR on Autophagy
As in the case of the daf-2 mutants, inactivation of TOR signaling in C. elegans also resulted in increased levels of autophagy, and suppression of autophagy resulted in the reversal of these lifespan-increasing effects (Hansen et al., 2008). Mechanistically, mTOR (while in the mTORC1 complex) has been shown to inhibit autophagy in two ways—via direct phosphorylation and inhibition of the autophagy-initiating kinase Ulk1 (Kim et al., 2011), and by phosphorylating transcription factor EB (TFEB) to prevent it from entering the nucleus where it can promote the expression of various autophagy and lysosomal genes (Martina et al., 2012).
.../...
F O R T H E R E S T O F T H E S T U D Y, P L E A S E V I S I T T H E S O U R C E .
.











































