http://www.bioscienc...npress/3174.pdf

The mitochondrial free radical theory of ageing – Where do we stand?
#1
Posted 12 April 2008 - 08:09 AM
#2
Posted 13 April 2008 - 09:54 PM
What is with it? for years I am wondering what happens, years after an article is published, all sight of it disappears, a few years later a very samiliar articles shows (for example tooth regeneration, been few years ago, now a new one) but never a therapy.
#3
Posted 14 April 2008 - 12:41 AM
It says May1st. How do they do that? Do they throw banana skins in the the flux capacitor and then .......whoosh......the future. Perhaps they should call the journal Futures in Bioscience instead of frontiers
sponsored ad
#4
Posted 14 April 2008 - 06:12 AM
I think the date is based on the paper journal, which has to get printed, bound, and shipped, so it's post-dated. An artifact of old technology.Thanks Shepard.
It says May1st. How do they do that? Do they throw banana skins in the the flux capacitor and then .......whoosh......the future. Perhaps they should call the journal Futures in Bioscience instead of frontiers
Thanks, Shepard. That's a great paper.
#5
Posted 17 April 2008 - 11:50 AM
"In summary, [...] there is, to date, no convincing evidence that oxidative damage to mtDNA is indeed extensive in vivo."
"While unphysiologically high mtDNA mutations levels are clearly pathogenic, their low abundance in normal animals raises questions regarding their functional relevance in the context of normal aging."
I haven't gone through all the papers they're citing though.
Bad news for MitoSENS? I guess Aubrey's reply would be something along the lines that it's not worth spending too much time trying to figure out the exact mechanisms and just fix it instead. But if the underlying assumptions for his engineering approach are kindof shakey...
I'm really, really curious how well mice with the free radical-problem fixed (somehow) would do in the m-prize.
#6
Posted 17 April 2008 - 06:47 PM
A few years ago while I was in school I remember my professor showing a image of older mitochondria wall that had been damaged by something. I think at the time he thought the culprit was actually O2 that we consume causes damage over a long time on the wall membrane of the Mitochondria. Also the Mitochondria doesn't really have a repair mechanism? Also O2 is a diradical and maybe over time to could damage a cell wall.Hmm, this article seems to be blowing some pretty big holes in the mtFRTA. I thought if one thing was clear about the biology of aging it's that free radicals/ROS are a big culprit, and it would make sense that mtDNA is their primary damage site. But..
"In summary, [...] there is, to date, no convincing evidence that oxidative damage to mtDNA is indeed extensive in vivo."
"While unphysiologically high mtDNA mutations levels are clearly pathogenic, their low abundance in normal animals raises questions regarding their functional relevance in the context of normal aging."
I haven't gone through all the papers they're citing though.
Bad news for MitoSENS? I guess Aubrey's reply would be something along the lines that it's not worth spending too much time trying to figure out the exact mechanisms and just fix it instead. But if the underlying assumptions for his engineering approach are kindof shakey...
I'm really, really curious how well mice with the free radical-problem fixed (somehow) would do in the m-prize.
I don't have any papers but one of the few things I remember out of school
#7
Posted 17 April 2008 - 09:19 PM
As for the repair mechanism, the authors of this paper don't seem to be too convinced the mito's are helpless against DNA damage. I also recall something about mtDNA polymerase having better proofreading capability than nDNA polymerases, so I guess that's at least something.
Edited by Bram, 17 April 2008 - 09:20 PM.
#8
Posted 17 April 2008 - 09:53 PM
Cheers, Aubrey
#9
Posted 17 April 2008 - 09:54 PM
O2, dioxide? The normal state of oxygen as we breathe it in, causing problems for the mito membranes? That's new for me.. I thought it was stuff like superoxide and hydroxyl formed as a byproduct during oxidative phosphorylation from O2 that was causing the problems. Can you remember anything else about it?
I can't seem to find much about regular O2 in mitos with a quick search.
As for the repair mechanism, the authors of this paper don't seem to be too convinced the mito's are helpless against DNA damage. I also recall something about mtDNA polymerase having better proofreading capability than nDNA polymerases, so I guess that's at least something.
http://www.meta-synt.../diradical.html
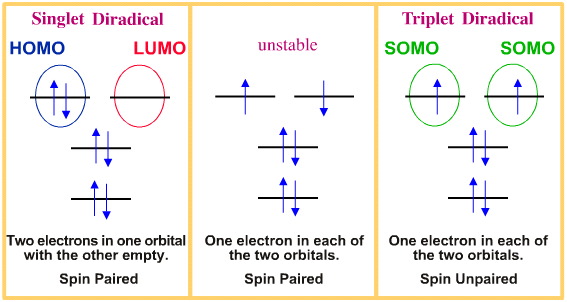
Subcellular organelles:
Organelles such as mitochondria, chloroplasts, microsomes, peroxisomes and nuclei have been shown to generate O2·⁻ and this is easily demonstrated after the endogenous superoxide dismutase has been washed away (Asada and Kiso, 1973). Mitochondria are the main cellular organelle for cellular oxidation reactions and the main source of reduced oxygen species in the cell. The leaks in mitochondrial electron transport system allow O2 to accept a single electron forming O2·⁻ (Kalra et al. 1994; Haliwell, 1995). It has been shown that superoxide production by the mitochondria increases in two conditions; either when the oxygen concentration is greatly increased or when the respiratory chain becomes fully reduced (as happens during ischemia).
http://www.pubmedcen...I0524408.f2.jpg
Maybe over time it contributes to mito damage?
#10
Posted 13 March 2009 - 11:06 PM
MIT biologist Leonard Guarente believes the theory on oxygen radicals is wrong. He believes aging is dependant on a gene that makes a protein called Sir2 and a high respiration rate. When SIR2 goes up, longevity happens.
More Info on Sir2: http://web.mit.edu/b...yeast/sir2.html
One might also note that it has been demonstrated that the brain can switch to B-hydroxybutyrate and acetoacetate as the predominant fuel for brain metabolism instead of glucose and the results are longer life & better function.
So if you want humans to live for hundreds of years you might increase SIR2 and switch the brain fuel to B-hydroxybutyrate and acetoacetate instead of glucose. You will also have to keep the cells clean of garbage to ensure clean copies.
Regards,
Jordan O'Hara
Edited by curezone, 13 March 2009 - 11:06 PM.
#11
Posted 15 March 2009 - 04:53 PM
MIT biologist Leonard Guarente believes the theory on oxygen radicals is wrong. He believes aging is dependant on a gene that makes a protein called Sir2 and a high respiration rate. When SIR2 goes up, longevity happens.
Yeast Sir2 and its human analogue Sirt1 are autophagy promoters. Autophagy recycles cell components including mitos. When the mito population is being consumed by autophagy, mito reproduction in the remaining mitos must kick in to maintain the mito population levels. Reproduction by binary fission is the most elaborate mito function and thus the most likely to fail if the mito DNA is extensively damaged. It is this selection pressure that maintains the youth of mitos. Just like bacteria, the individuals who sustain harmful mutations are weeded out.
With this mechanism, bacteria have survived for billions of years. Indeed every bacterium alive today is billions of years old since it has as much claim on the identity of its parent as does its sister. Mitos are just degenerate bacteria. They have about ten copies of their genome in ten rings of DNA. With such redundancy, they can sustain a lot of DNA damage and remain functional. During reproduction, damaged rings may even be unable to make a copy of themselves and be weeded out. The daughter mitos could then be free of the DNA damage of the parent.
1 user(s) are reading this topic
0 members, 1 guests, 0 anonymous users