Very interesting finding Rior.

In this thread:
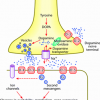
http://www.longecity...timulates-bdnf/Computethis suggest using ALCAR to increase Acetyl-CoA, which is responsible for the conversion of serotonin into N-acetylserotonin. So perhaps by compining both Duloxetine and ALCAR we shall get a synergetic effect.
As for the none SRI, I found this reports:
https://en.wikibooks...tic_StimulationAllegations that ECT may cause brain damage have been consistently refuted. In fact, it seems that ECT may stimulate an increased production of neurotrophic growth factors such as brain derived neurotrophic factor (BDNF) causing migration and proliferation of progenitor cells and growth of new neurons in the hippocampus. These findings are consistent with evidence of similar effects of various other antidepressant treatments and may be the final common pathway of the antidepressant effects.
https://www.ncbi.nlm...pubmed/23201339Magnetic stimulation modulates structural synaptic plasticity and regulates BDNF-TrkB signal pathway in cultured hippocampal neuron
Ma J, Zhang Z, Su Y, Kang L, Geng D, Wang Y, Luan F, Wang M, Cui H.
Source
Deparment of Anatomy, Hebei Medical University, Shijiazhuang 050017, Hebei, PR China; Hebei Key Laboratory for Brain Aging and Cognitive Neuroscience, The First Hospital of Hebei Medical University, Shijiazhuang 050031, Hebei, PR China; Hebei Institute of Physical Education, Shijiazhuang 050041, Hebei, PR China.
Abstract
Repetitive transcranial magnetic stimulation (rTMS) is a neuropsychiatric tool that can be used to investigate the neurobiology of learning and cognitive function. Few studies have examined the effects of low frequency (⩽1Hz) magnetic stimulation (MS) on structural synaptic plasticity of neurons in vitro, thus, the current study examined its effects on hippocampal neuron and synapse morphology, as well as synaptic protein markers and signaling pathways. Similarly, both intensities of low frequency magnetic stimulation (1Hz) activated brain-derived neurotrophic factor (BDNF) and tropomyosin-related kinase B (TrkB) pathways, including the pathways for mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) and for phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt). Specifically, low intensity magnetic stimulation (LIMS, 1.14Tesla, 1Hz) promoted more extensive dendritic and axonal arborization, as well as increasing synapses density, thickening PSD (post synaptic density) and upregulation of synaptophysin (SYN), growth associated protein 43 (GAP43) and post synaptic density 95 (PSD95). Conversely, high intensity magnetic stimulation (HIMS, 1.55Tesla, 1Hz) appeared to be detrimental, reducing dendritic and axonal arborization and causing apparent structural damage, including thinning of PSD, less synapses and disordered synaptic structure, as well as upregulation of GAP43 and PSD95, possibly for their ability to mitigate dysfunction. In conclusion, we infers that low frequency magnetic stimulation participates in regulating structural synaptic plasticity of hippocampal neurons via the activation of BDNF-TrkB signaling pathways.
Copyright © 2012. Published by Elsevier Ltd.
https://www.ncbi.nlm...pubmed/21795553 Repetitive transcranial magnetic stimulation enhances BDNF-TrkB signaling in both brain and lymphocyte.
Wang HY, Crupi D, Liu J, Stucky A, Cruciata G, Di Rocco A, Friedman E, Quartarone A, Ghilardi MF.
Source
Department of Physiology, Pharmacology & Neuroscience, Sophie Davis School of Biomedical Education, The City University of New York Medical School, New York, New York 10031, USA. hywang@sci.ccny.cuny.edu
Abstract
Repetitive transcranial magnetic stimulation (rTMS) induces neuronal long-term potentiation or depression. Although brain-derived neurotrophic factor (BDNF) and its cognate tyrosine receptor kinase B (TrkB) contribute to the effects of rTMS, their precise role and underlying mechanism remain poorly understood. Here we show that daily 5 Hz rTMS for 5 d improves BDNF-TrkB signaling in rats by increasing the affinity of BDNF for TrkB, which results in higher tyrosine-phosphorylated TrkB, increased recruitment of PLC-γ1 and shc/N-shc to TrkB, and heightened downstream ERK2 and PI-3K activities in prefrontal cortex and in lymphocytes. The elevated BDNF-TrkB signaling is accompanied by an increased association between the activated TrkB and NMDA receptor (NMDAR). In normal human subjects, 5 d rTMS to motor cortex decreased resting motor threshold, which correlates with heightened BDNF-TrkB signaling and intensified TrkB-NMDAR association in lymphocytes. These findings suggest that rTMS to cortex facilitates BDNF-TrkB-NMDAR functioning in both cortex and lymphocytes.
https://www.ncbi.nlm...pubmed/21593336 Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: differential outcomes in anesthetized and awake animals.
Gersner R, Kravetz E, Feil J, Pell G, Zangen A.
Source
Department of Neurobiology, Weizmann Institute of Science, Rehovot 76100, Israel.
Abstract
Long-term effects of repetitive transcranial magnetic stimulation (rTMS) have been associated with neuroplasticity, but most physiological studies have evaluated only the immediate effects of the stimulation on neurochemical markers. Furthermore, although it is known that baseline excitability state plays a major role in rTMS outcomes, the role of spontaneous neural activity in metaplasticity has not been investigated. The first aim of this study was to evaluate and compare the long-term effects of high- and low-frequency rTMS on the markers of neuroplasticity such as BDNF and GluR1 subunit of AMPA receptor. The second aim was to assess whether these effects depend on spontaneous neural activity, by comparing the neurochemical alterations induced by rTMS in anesthetized and awake rats. Ten daily sessions of high- or low-frequency rTMS were applied over the rat brain, and 3 d later, levels of BDNF, GluR1, and phosphorylated GluR1 were assessed in the hippocampus, prelimbic cortex, and striatum. We found that high-frequency stimulation induced a profound effect on neuroplasticity markers; increasing them in awake animals while decreasing them in anesthetized animals. In contrast, low-frequency stimulation did not induce significant long-term effects on these markers in either state. This study highlights the importance of spontaneous neural activity during rTMS and demonstrates that high-frequency rTMS can induce long-lasting effects on BDNF and GluR1 which may underlie the clinical benefits of this treatment in neuroplasticity-related disorders.