.
Abstract
Genomic destabilisation is associated with the induction of mutations, including those in cancer-driver genes, and subsequent clonal evolution of cells with abrogated defence systems. Such mutations are not induced when genome stability is maintained; however, the mechanisms involved in genome stability maintenance remain elusive. Here, resveratrol (and related polyphenols) is shown to enhance genome stability in mouse embryonic fibroblasts, ultimately protecting the cells against the induction of mutations in the ARF/p53 pathway. Replication stress-associated DNA double-strand breaks (DSBs) that accumulated with genomic destabilisation were effectively reduced by resveratrol treatment. In addition, resveratrol transiently stabilised the expression of histone H2AX, which is involved in DSB repair. Similar effects on the maintenance of genome stability were observed for related polyphenols. Accordingly, we propose that polyphenol consumption can contribute to the suppression of cancers that develop with genomic instability, as well as lifespan extension.
Introduction
Most cancers are associated with genomic instability, which can be categorised as chromosomal instability or microsatellite instability (MSI)1. Genomic destabilisation is a major cause of mutations, including those in cancer-driver genes, and can lead to clonal evolution of cells with abrogated defence systems, such as those containing mutations in the ARF/p53 pathway2. Genome stability maintenance would likely prevent the formation of mutations and suppress cancer development; however, it is still unclear if genome stability can be maintained in vivo and whether this process can indeed suppress the occurrence of cancer.
Genomic instability is caused by the erroneous repair of DNA double-strand breaks (DSBs); paradoxically, the DNA repair systems of most cancers that develop with genomic instability are genetically normal3. The mechanisms by which normal cells accumulate DSBs remain unclear, but DSBs widely accumulate in pre-cancerous cells and are accompanied by genomic instability2,4,5,6. In vitro, replication stress-associated DSBs and the associated genomic instability are observed in cells subjected to aberrant growth stimulation2 or overexpression of oncogenes such as c-Myc and E2F16,7,8.
Possibly reflecting the correlation between cancer development and age, DSBs accumulate with age in vivo and with cultivating passages in vitro9, suggesting that ageing cells are defective in DSB repair. DSB repair deficiency is probably related at least in part to a reduction in the level of H2AX. This histone mediates DSB repair and is required for genome stability maintenance, and H2AX expression levels are attenuated when the growth rate of normal cells slows down10,11. In fact, such cells are defective in repairing replication stress-associated DSBs10, although they are still able to repair DSBs caused by γ-rays because H2AX is transiently stabilised under these conditions12.
A number of animal studies have shown that regular polyphenol consumption can contribute to cancer suppression in association with lifespan extension13,14,15,16,17,18. Such cancer-suppressive effects of polyphenols have been reported for a wide variety of cancers that generally arise with genomic instability at advanced ages, such as skin19, prostate20, colon21 and breast cancers22. It is possible that the anti-cancer and anti-aging effects of polyphenols are related to their positive effects on genome integrity.
In this study, we examined the effects of polyphenols on genome stability maintenance, DSB repair and genomic instability-associated cancer suppression. We found that resveratrol, a polyphenol found in red wine, and related polyphenols maintain genome stability by inducing DSB repair, thereby contributing to the suppression of cancer that develops with genomic instability.
Results
Resveratrol contributes to genome stability
To examine the effects of resveratrol on genome stability, we monitored the immortalisation of mouse embryonic fibroblasts (MEFs) (Fig. 1a). When cultivated under the ‘3T3 protocol’, MEFs initially show serial proliferation, but then undergo growth-arrested senescence, and subsequently immortalise with genomic instability (tetraploidy)8,10 and mutations in the ARF/p53 pathway11,23. As expected, MEFs grown under the standard 3T3 protocol (Std-3T3) immortalised with tetraploidy, but MEFs that were regularly treated with 2.5 μM resveratrol (Resv-3T3 protocol) displayed continuous genome stability and were protected against immortalisation (Fig. 1a,b). Supporting these results, the percentages of cells with two nuclei or micronuclei, the levels of which increase during genomic destabilisation, were lower for the Resv-3T3 group than for the Std-3T3 group (Supplementary Fig. S1a). The induction of aberrant nuclei by γ-ray irradiation was also reduced in the presence of resveratrol, although the differences between the control cells and resveratrol-treated cells were not statistically significant (Supplementary Fig. S1b). These results indicate that continuous resveratrol treatment contributes to genome stability maintenance. Given that MEFs generally immortalise with abrogation of the ARF/p53-dependent barrier11,23, resveratrol may suppress the induction of mutations in cancer-driver genes via the maintenance of genome stability.
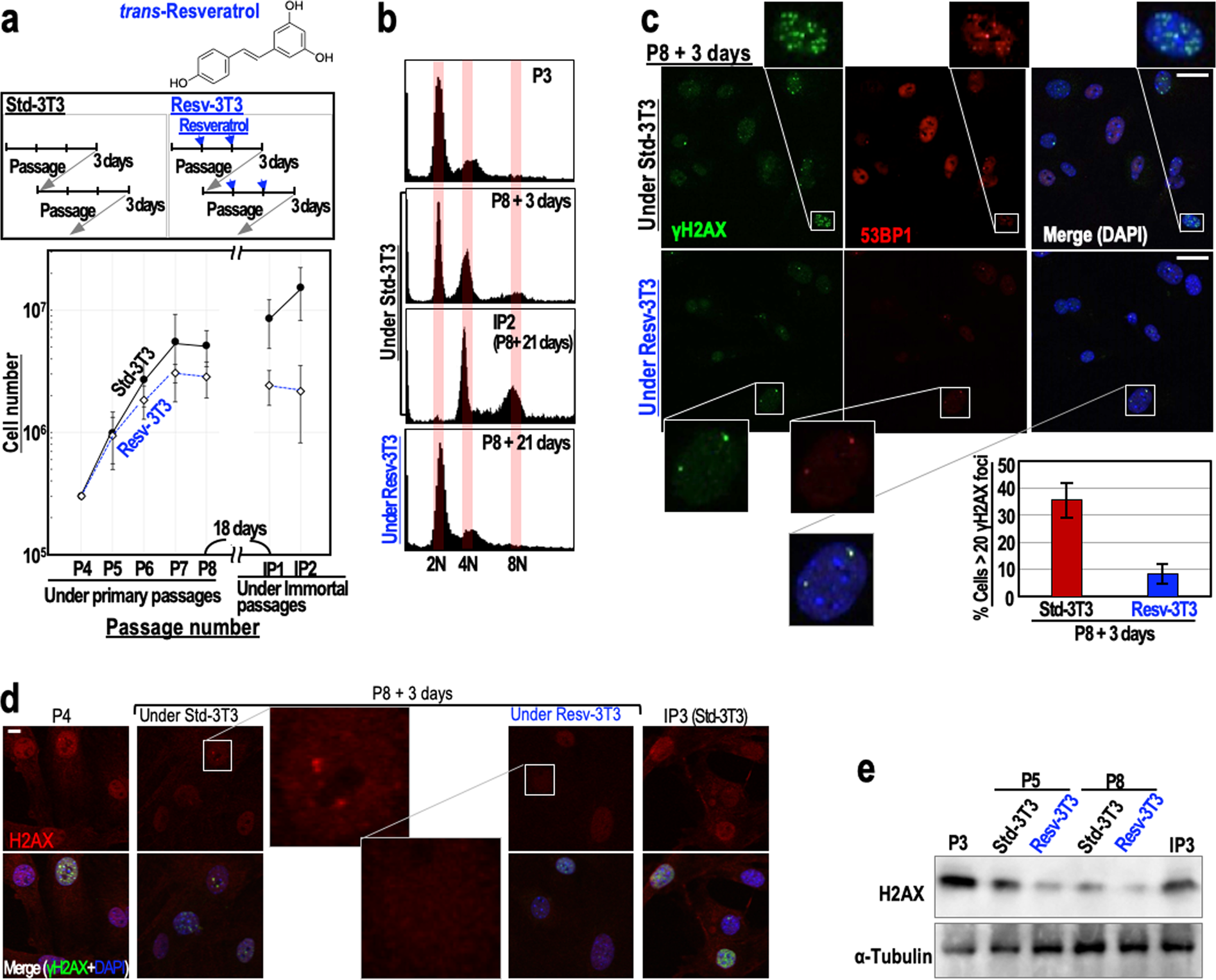
Figure 1. Regular resveratrol treatment maintains genome stability and suppresses DSB accumulation. (a) The immortalisation of MEFs cultivated under the Std-3T3 or Resv-3T3 protocol as indicated. In the Resv-3T3 protocol, the cells were treated with 2.5 μM resveratrol regularly. In the lower graph, data are represented as the mean ± s.d. (n = 3 biologically independent experiments). (b) The effects of cultivation of MEFs under the Std-3T3 or Resv-3T3 protocol on chromosomal instability (tetraploidy). © Statuses of the γH2AX and 53BP1 foci in MEFs at a growth-arrested stage (P8 + 3 days) following cultivation under the Std-3T3 or Resv-3T3 protocol. The numbers of γH2AX foci were quantified and data are represented as the mean ± s.d. (n = 3 biologically independent experiments). (d,e) Immunostaining (d) and immunoblot (e) analyses of H2AX in quiescent MEFs grown under the Std-3T3 or Resv-3T3 protocol. (c,d) Scale bars, 10 μm.
Genomic destabilisation (tetraploidisation) in MEFs is generally triggered by replication stress-associated DSBs that accumulate under the Std-3T3 protocol, in association with cellular stress caused by continuous growth acceleration. Since our initial experiment suggested that regular resveratrol treatment can suppress genomic destabilisation, we examined the accumulation of DSBs in passage 8 (P8) MEFs grown under the Std-3T3 and Resv-3T3 protocols, by counting the numbers of γH2AX/53BP1 foci. As expected, the number of γH2AX/53BP1 foci in MEFs grown under the Resv-3T3 protocol was markedly smaller than that in MEFs grown under the Std-3T3 protocol (Fig. 1c). This finding is consistent with the observed genome stabilising effect of resveratrol described above, and suggests that regular resveratrol treatment is associated with the reduction of DSBs and the neutralisation of replication stress.
Normal cells generally undergo growth arrest after serial proliferation. At this stage, two different cellular states can be discriminated: (1) a senescent state associated with γH2AX foci accumulation and an increased risk of genomic destabilisation, and (2) a quiescent state associated with down-regulated H2AX expression and maintenance of genome stability24. Therefore, we compared the expression levels of H2AX in MEFs grown under the Std-3T3 and Resv-3T3 protocols. As expected, an immunostaining analysis revealed that the H2AX signal was substantially lower in the Resv-3T3 cells than in the Std-3T3 cells at the growth-arrested stage (P8 + 3 days) (Fig. 1d). A western blotting analysis confirmed that, as seen in quiescent cells, H2AX expression was down-regulated in both cell groups at the growth-arrested stage, but the reduction was larger for the resveratrol-treated cells than for those grown under the standard protocol (Fig. 1e). Together with the observed reduction in γH2AX/53BP1 foci formation following resveratrol treatment, these findings indicate that resveratrol contributes to DSB reduction and genome stability maintenance by inducing a quiescent state with down-regulated H2AX levels.
Resveratrol enables DSB repair through transient induction of H2AX
After P8, MEFs are at an increased risk of genomic destabilisation due to a deficiency in the repair of DSBs caused by the replication stress that arises during cultivation under Std-3T3 conditions. Although it is unclear how normal cells become defective in DSB repair after serial proliferation, the process is thought to involve a reduction in H2AX levels10,11. It is possible that the repair of DSBs in such cells occurs via transient up-regulation of H2AX, as occurs after γ-ray irradiation of cells12. To test this hypothesis, we performed a 24 hr time-course analysis of H2AX levels in MEFs treated with 5 µM resveratrol (Fig. 2a,b). As expected, H2AX was transiently induced after resveratrol treatment of P8 MEFs, with the peak induction at 6 hr post-treatment. In addition, H2AX transiently induced by resveratrol was efficiently incorporated into chromatin but its level decayed within 24 hr (Fig. 2c).
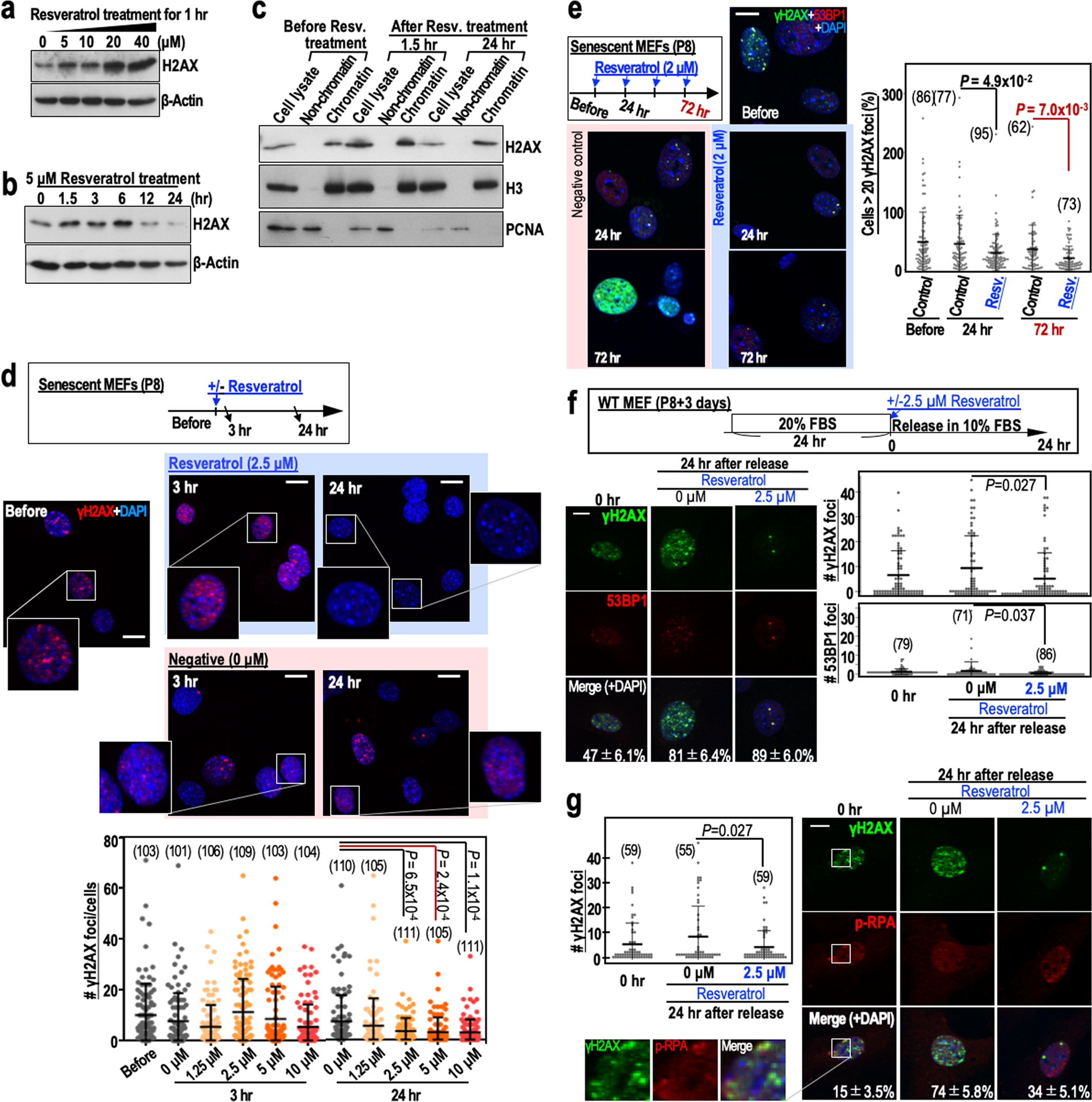
Figure 2. Resveratrol treatment of MEFs transiently stabilises H2AX, leading to reduced numbers of γH2AX foci. (a) H2AX expression in senescent MEFs (passage 8) treated with different concentrations of resveratrol. The dose-dependent effects of resveratrol were assessed 1 hr after treatment. (b) The time-dependent effects of 5 μM resveratrol on H2AX expression in senescent MEFs (passage 8). © H2AX expression in senescent MEFs (passage 8) treated with resveratrol (5 μM) and then fractionated into the chromatin and non-chromatin fractions before and 1.5 and 24 hr after treatment. Histone H3 (H3) and PCNA were used as controls. (d) Immunofluorescence analyses examining the effect of resveratrol (2.5 μM) on the number of γH2AX foci in senescent MEFs (n numbers are indicated in the graph). The dose-dependent effects of resveratrol were assessed 3 and 24 hr after treatment. (e) The effects of multiple resveratrol treatments (2 μM) on the number of γH2AX foci in senescent MEFs. Reductions in the number of γH2AX foci were assessed 24 hr (single treatment) and 72 hr (three treatments) after resveratrol exposure. Data are represented as the mean ± s.d. (n = 3 biologically independent experiments). (f,g) The effects of resveratrol (2.5 μM) on the numbers of merged γH2AX/53BP1 (f) and γH2AX/p-RPA (g) foci in senescent MEFs. The MEFs were treated as shown in the workflow (f). γH2AX, 53BP1 and p-RPA were detected by immunofluorescence (n numbers are indicated in the graph). The percentages of the γH2AX foci that merged with 53BP1 or p-RPA foci (mean ± s.e.) are indicated in each image. Data in the graphs are represented as the mean ± s.d. Scale bars, 10 μm. P-values were calculated by two-tailed Welch’s t-tests.
To examine its effect on DSB repair, we treated P8 MEFs with different concentrations of resveratrol and monitored γH2AX foci status. As expected, the number of γH2AX foci was reduced significantly 24 hr after treatment of MEFs with 2.5 µM resveratrol (Fig. 2d). In addition, the number of γH2AX foci was reduced further by multiple resveratrol treatments (Fig. 2e). The γH2AX foci largely merged with 53BP1 foci, which accumulate at DSB sites (Fig. 2f), and with phosphorylated RPA32 (p-RPA at Ser33) foci, which arise in association with replication stress (Fig. 2g). The numbers of these foci were reduced in the presence of resveratrol (Fig. 2f), suggesting efficient repair of DSBs in the presence of resveratrol.
Resveratrol may modulate multiple biological pathways and may induce apoptosis at high doses25. To compare the conditions that lead to DSB repair and apoptosis, the effects of three concentrations of resveratrol (2.5, 25 and 250 μM) on multiple cell types were examined after pre-treatment of cells with 0.25 mM hydroxyurea to weakly induce replication stress. As expected, the level of cleaved caspase-3, a marker of apoptosis induction, was increased after treatment of MEFs (P4), HUC-F2 cells (P9) and WI38 cells (P6) with 250 μM resveratrol, and after treatment of HeLa cells with 25 μM resveratrol (Supplementary Fig. S2a). Under these conditions, the numbers of γH2AX foci were not increased prior to the induction of apoptosis (Supplementary Fig. S2b; see 25 μM for HeLa and 250 μM for MEFs), implying the separation of apoptosis induction from damage responses. Notably, these findings indicate that the concentration of resveratrol that leads to DSB reduction is much lower than that required for the induction of apoptosis.
Normal cells treated with hydroxyurea usually undergo growth arrest and hence show only a limited response to damage26. Therefore, we also examined the effect of resveratrol on the numbers of replication stress-associated DSBs 24 hr after γ-ray irradiation (2 Gy) of MEFs, HeLa cells and WI38 cells (Supplementary Fig. S3a), because replication stress-associated DSBs accumulate after the repair of DSBs that are directly caused by γ-ray exposure27. As expected, the number of γH2AX foci that merged with p-RPA (Supplementary Fig. 3b) and 53BP1 was reduced significantly in the presence of 2.5 μM resveratrol (Supplementary Fig. S3a). Furthermore, the numbers of γH2AX foci and 53BP1 foci in MEFs that were γ-ray irradiated with 1 or 5 Gy were also reduced significantly in the presence of 2.5 μM resveratrol (Supplementary Fig. S3c). Based on these findings, we concluded that replication stress-associated DSBs are reduced by treatment of cells with relatively low concentrations of resveratrol.
Resveratrol-related polyphenols contribute to genome stability
A number of studies have reported potential benefits of polyphenol consumption on cancer suppression and longevity28,29,30. Therefore, we examined the genome stabilising effects of chlorogenic acid, a polyphenol found in coffee, and melinjo resveratrol, a mixture of multiple resveratrol-associated polyphenols produced in melinjo seeds. Similar to resveratrol (Fig. 1), chlorogenic acid (2.5 μM) and melinjo resveratrol (0.5 μg/ml) inhibited the immortalisation of MEFs (Fig. 3a) and promoted genome stability (Fig. 3b). In addition, as seen for resveratrol, chlorogenic acid and melinjo resveratrol caused a transient induction of H2AX (Fig. 3c,d) and a reduction in the number of γH2AX foci (Fig. 3e). These findings suggest that the broad health benefits of these polyphenols could be due to the maintenance of genome stability, which primarily occurs via the reduction of DSBs.
.../...
.











































