.
O P E N A C C E S S S O U R C E : International Journal of Molecular Sciences
Abstract
Age-related hearing loss (ARHL) is an irreversible, progressive neurodegenerative disorder and is presently untreatable. Previous studies using animal models have suggested mitochondrial damage and programmed cell death to be involved with ARHL. Thus, we further investigated the pathophysiologic role of mitochondria and necroptosis in aged C57BL/6J male mice. Aged mice (20 months old) exhibited a significant loss of hearing, number of hair cells, neuronal fibers, and synaptic ribbons compared to young mice. Ultrastructural analysis of aged cochleae revealed damaged mitochondria with absent or disorganized cristae. Aged mice also showed significant decrease in cochlear blood flow, and exhibited increase in gene expression of proinflammatory cytokines (IL-1β, IL-6, and TNF-α), receptor-interacting serine/threonine-protein kinase 1 and 3 (RIPK1 and RIPK3) and the pseudokinase mixed-lineage kinase domain-like (MLKL).
Immunofluorescence (IF) assays of cytochrome C oxidase I (COX1) confirmed mitochondrial dysfunction in aged cochleae, which correlated with the degree of mitochondrial morphological damage. IF assays also revealed localization and increased expression of RIPK3 in sensorineural tissues that underwent significant necroptosis (inner and outer hair cells and stria vascularis). Together, our data shows that the aging cochlea exhibits damaged mitochondria, enhanced synthesis of proinflammatory cytokines, and provides new evidence of necroptosis in the aging cochlea in in vivo.
1. Introduction
Approximately one in five people over the age of 50 has imperfect hearing, and almost half of those aged over 65 years have hearing difficulties [1,2,3,4]. Presbycusis, also termed age-related hearing loss (ARHL), is an irreversible hearing impairment associated with aging due to limited repair capacity of sensorineural tissues in the cochlea. Unfortunately, there is no effective cure for the patients, and future treatment development is still questionable due to lack of mechanistic insight [5]. In order to identify pathologic changes in the aged cochleae, we utilized C57BL/6J mice to investigate the pathophysiology of ARHL, as this strain displays accelerated, high-frequency hearing loss by 3–6 months of age and profound hearing impairment by 15 months of age [6,7,8,9,10,11].
Mitochondria are involved in the metabolic dysregulation associated with ARHL pathology. Morphologically, damage is apparent in the outer hair cells (OHCs) of animal ARHL models [10,12,13]. Mitochondria are the principal source of reactive oxygen species (ROS), the production of which is closely associated with ARHL progression. Antioxidants alleviate the deleterious effects of ROS and effectively treat oxidative stress-related diseases in an animal model of ARHL [14]. As mitochondria play key roles in both the respiratory chain and cell death, animal models of ARHL often exhibit defects in mitochondrial enzyme activities and mitochondrial-mediated apoptosis. Idh2-knockout mice exhibit accelerated ARHL progression, accompanied by a profound loss of hair cells and spiral ganglion neurons (SGNs), an increase in oxidative damage, and increased apoptotic cell death [15]. The mitochondrial proapoptotic BCL2-antagonist/killer 1 (Bak) gene mediates ARHL in C57BL/6J mice by enhancing mitochondrial fission and cellular apoptosis, both of which are systematic responses to oxidative stress [10].
Neuronal cell death occurs through various pathways in sensorineural tissue, leading to hearing impairment [2,5,10,16]. Necroptosis is a programmed cell death that exhibits necrosis-like morphological characteristics. Necroptosis is activated by the receptor-interacting protein (RIP) homology interaction motif (RHIM), and is mediated by proteins such as RIP3 and the mixed-lineage kinase domain-like (MLKL) protein [17]. Unlike apoptosis, necroptosis permeabilizes both intra- and extracellular membranes, releasing cellular and organelle contents into the extracellular medium and inducing inflammation. Necroptosis plays an important role in the pathogenesis of aging, neurodegenerative diseases, and hearing impairments including cisplatin- and aminoglycoside-induced ototoxicity and noise-induced hearing loss [17,18]. However, to the best of our knowledge, cochlear necroptosis has not been described in ARHL animal models. As successful modulation of cell death pathways may lead to potential development of clinically applicable drugs, we aimed to investigate the pathophysiology of ARHL by focusing on mitochondrial damage and necroptosis.
2. Materials and Methods
2.1. Experimental Animals and Design
All animal experiments were approved by Chungnam National University, Institutional Animal Care and Use Committee (IACUC, 9 January 2016). All animal care and use was conducted in accordance with the Guide for the Care and Use of Laboratory Animals. C57BL/6J male mice, aged 2 months or 20 months, were used in this study.
2.2. Auditory Brainstem Response (ABR)
ABR thresholds at frequencies between 4 and 32 kHz, and click sounds, were obtained separately from both ears as described previously [19]. The TDT System-3 (Tucker Davis Technologies, Gainesville, FL, USA) hardware and software were used to obtain the ABRs. The stimuli were computer-generated tone pips. The animals were anesthetized with intramuscular injection of zolazepam HCl 40 mg/kg (Zoletil, Virbac Animal Health, Carros, France) and xylazine 10 mg/kg (Rompun, Bayer Animal Health, Monheim, Germany). Subcutaneous needle electrodes were placed around the skull vertex and both infraauricular areas. Tone bursts, with a duration of 4 ms and rise-fall time of 1 ms at frequencies of 4, 8, 16, and 32 kHz, were used, in addition to clicks.
The sound intensity was varied in 10 dB increments for the tone burst sounds and in 5 dB increments for the click and tone burst sounds close to the threshold. The contralateral ear was not masked because the stimuli were transmitted through a sealed earphone. The waveforms were analyzed using a custom program (BioSig RP, ver. 4.4.1; Tucker Davis Technologies) with the researcher blinded to the treatment group. Threshold was defined as the lowest stimulus intensity to evoke a wave III response > 0.2 μV.
2.3. Tissue Preparation and Immunofluorescence
Samples from mice aged 2 and 20 months were collected. Cochlear tissues were obtained to localize inner and outer hair cells and neuronal fibers as previously described [20,21]. Briefly, tissues were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 1 h at room temperature to remove the cochlear bony walls and lateral wall tissues and separated individual cochlear turns. The remaining cochlear tissues were prepared within immunofluorescence. Tissues were blocked in 0.3% Triton X-100 (Sigma-Aldrich, St. Louis, MO) in 5% normal goat serum (Vector Laboratories, Burlingame, CA) for 1 h, then incubated with rabbit anti-myosin VIIa primary antibody (Proteus BioSciences, Ramona, CA)—Alexa Fluor 488 Phalloidin (A12379; Invitrogen-Molecular Probes, Eugene, OR); rabbit anti-CtBP2 primary antibody (BD Biosciences)—Alexa Fluor 594 Phalloidin (A11034; Invitrogen-Molecular Probes, Eugene, OR); chicken anti-NF-H primary antibody (Millipore)—Alexa Fluor 488 Phalloidin (A11039; Invitrogen-Molecular Probes, Eugene, OR); mouse anti-COX1 (also known as MTCO1) primary antibody (Invitrogen); rabbit anti-RIPK3 primary antibody (Novus Biologicals); or Hoechst33342 (H3570; Invitrogen-Molecular Probes, Eugene, OR) at a concentration of 1:200 in blocking solution overnight at 4 °C (1:1000 for Hoechst33342 for 1 min). After rinsing six times in PBS for 10 min, the tissues were incubated with each secondary antibody at a concentration of 1:200 in PBS for 2 h. The specimens were mounted on glass slides using Crystalmount (Biomeda, Foster City, CA) and observed under an epifluorescence microscope (Zeiss Axio Scope A1; Zeiss, Oberkochen, Germany) with a digital camera.
2.4. Transmission Electron Microscopy (TEM)
The decalcificated mouse cochlea were prefixed immediately in 2.5% glutaraldehyde–2% paraformaldehyde in 0.15 M sodium cacodylate buffer (pH 7.4) for 2 h at 4 °C. After washing with sodium cacodylate buffer, tissue samples were postfixed in 2% osmium tetroxide–1.5% ferrocyanide in 0.15M cacodylate buffer (pH 7.4) for 1 h. Then, samples were incubated with 1% TCH for 30 min and treated with 2% OsO4 for 30 min. Subsequently, samples were En bloc stained with 1% uranyl acetate overnight at 4 °C and lead citrate for 30 min at 60 °C. The tissues were then embedded in Epon 812 mixture after dehydration in an ethanol and propylene oxide series. Polymerization was conducted with pure resin at 70 °C for 24 h. Sections (200 nm) were obtained with an ultramicrotome (Ultra Cut-UCT, Leica, Vienna, Austria) and then collected on 100 mesh copper grids. The sections were visualized using conventional TEM (JEM-1400Plus) at 120 kV and Bio-HVEM (JEM-1000BEF, JEOL, Tokyo, Japan) at 1000 kV. The sections were visualized using Bio-HVEM system (JEM-1400Plus at 120 kV and JEM-1000BEF at 1000 kV, JEOL, JAPAN).
2.5. Measurement of Cochlear Blood Flow
The left tympanic bulla of each mouse was exposed and opened under anesthesia. After the mouse was placed on the stereotaxic instrument, the cochlear blood flow was measured using a 0.1 mm diameter laser Doppler probe placed over the lateral wall of the cochlea. Cochlear blood flow was determined from an intensity oscillation that was translated from the frequency of the oscillation produced by the Doppler frequency shift of the red blood cells in the left tympanic bulla, using a Laser Doppler Flowmeter (Transonic Systems, Ithaca, NY, USA). Each intensity oscillation was measured separately, and relative cochlear blood flow was reported as the ratio of the control (pre) value to the postnoise exposure value.
2.6. Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
Quantitative RT-PCR was performed as previously described [19,22]. Briefly, tissues were collected and frozen immediately in liquid nitrogen and homogenized. Total RNA was extracted with TRIzol reagent (Thermo Fisher Scientific, Waltham, MA USA) according to the manufacturer’s protocol. RNA was quantified using a Nano drop (Nano Drop Technologies, Wilmington, DE). cDNA was produced using the cDNA synthesis kit (Roche, Branchburg, NJ, USA). Real time PCR was performed on a CFX Connect Real-Time PCR Detection System (BioRad, Des Plaines, IL, USA) by using a reaction mixture with SYBR Green as the fluorescent dye (Applied Biosystems, Waltham, Massachusetts, USA), a 1/10 vol of the cDNA preparation as template, and 250 nM of each primer (Realtime primers, PA). The fold change in the target gene relative to endogenous control gene (glyceraldehyde 3-phosphate dehydrogenase, GAPDH) was determined by: fold changeXX = 2−Δ(ΔCT) where ΔCT = CT,target gene − CT,GAPDH and Δ(ΔCT) = ΔCT,Aged cochlea − ΔCT,Young cochlea [23].
2.7. Image Processing and Statistical Analysis
Adobe Photoshop CS6 was used for adjustment of image contrast, superimposition of images, and colorization of monochrome fluorescence images. An unpaired Student’s t-test was used for all comparisons. A p-value < 0.05 was significant in each case. All tests were performed using GraphPad Prism 6.
3. Results
3.1. Auditory Brainstem Response Thresholds and Histopathologies of Young and Aging Cochleae
We first evaluated age-related functional impairment of hearing in male C57BL/6J mice aged 2 or 20 months. As shown in Figure 1, 20-month-old mice (n = 4) exhibited significantly increased auditory brainstem response (ABR) thresholds at 4, 8, 16, and 32 kHz and when click sounds were delivered (t-test, **** p < 0.0001). Whole-mount analyses of the auditory epithelium (Figure 2) confirmed that both inner hair cells (IHCs) (Figure 2A1–A3) and outer hair cells (OHCs) (Figure 2B1–B3) were more damaged in aging mice than in young mice. However, damage to the OHCs of all cochlear turns was more prominent than the damage to IHCs. Quantitative analyses of IHC (Figure 2C) and OHC (Figure 2D) survival indicated extensive loss of both cell types in aging mice; OHC loss was more marked than IHC loss (unpaired t-test, n = 3, * p < 0.05).
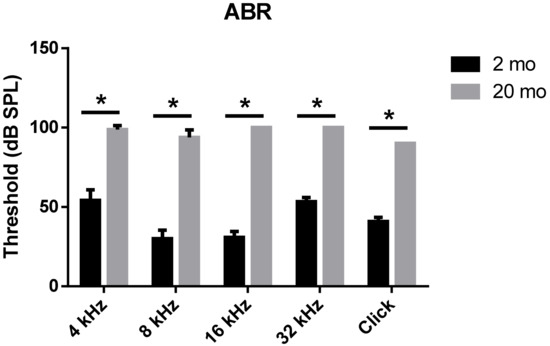
Figure 1. Aged mice displayed greater hearing loss as compared to young animals. ABR Table; 2-month- or 20-month-old male C57BL/6J mice. Aged mice exerted a significantly increased ABR threshold compared to young mice at all frequencies and click sound. The graph represents mean ± S.E.M. t-test. * p < 0.0001. n = 6, 2 months; n = 4, 20 months.
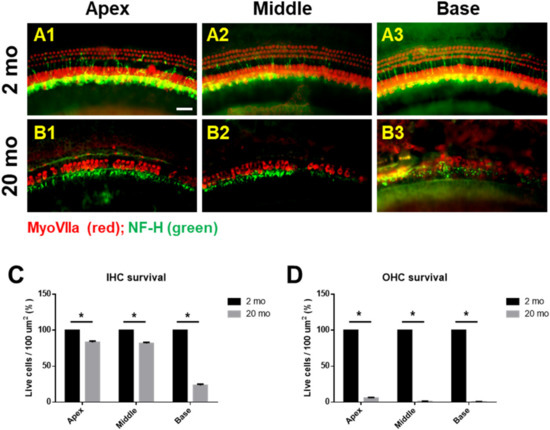
Figure 2. Cochlear hair cells are significantly lost in aged mice. Whole-mount preparations of the auditory epithelium in the 2 month-(A1–A3) and 20 month-old (B1–B3) mice. Tissues were stained for myosin VIIa (red) to visualize the hair cells and then photographed using epifluorescence. (A,B) OHCs were more noticeably destroyed on the middle and basal turns of the cochlea in the aged mice (B1–B3) compared to the young mice (A1–A3). (C,D) Quantitative analysis of hair cell survival on IHCs © and OHCs (D): apex, middle, and basal turns. A1 and B1, apical turn; A2 and B2, middle turn; A3 and B3, basal turn; OHC, outer hair cell; IHC, inner hair cell. Scale bar = 30 μm. All graphs represent mean ± S.E.M. * p < 0.05. Unpaired t-test. n = 3.
Next, whole mounts of the auditory epithelium were triple-stained with a Hoechst stain specific for nuclei, fluorescence-tagged antibodies against neurofilament-H (NF-H), and C-terminal binding protein 2 (CtBP2; a marker of synaptic ribbons), to evaluate synaptopathy. Figure 3A is a representative photograph. Hair cells (blue) and neuronal filaments (green) remained intact in young mice, consistent with the results shown in Figure 2, but the numbers of IHCs and OHCs (stained blue by Hoechst) were significantly reduced in aging mice. Neuronal filaments of the auditory epithelium (green, Figure 3A) were notably damaged in aging cochlea. The numbers of presynaptic marker counts (Figure 3B,C; CtBP2/10 inner hair cells) were significantly lower in aging mice than in young mice (unpaired t-test, ****p < 0.0001, n = 3), indicating severe synaptopathy and nerve fiber loss in aging cochleae.
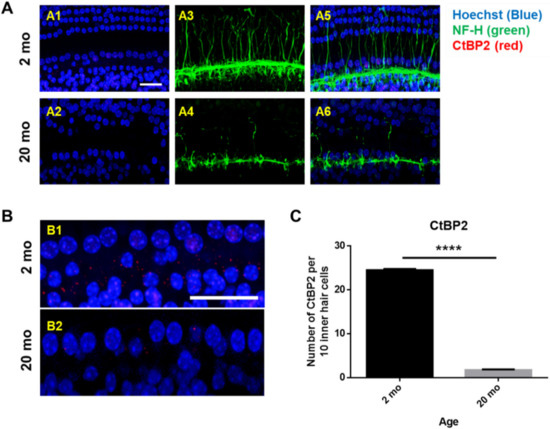
Figure 3. Severe synaptopathy and loss of nerve fibers were observed in aged mice. Whole-mounts of auditory epithelium were triple-stained with Hoechst (blue, nuclear marker, A1 and A2), NF-H (green, neuronal cell marker, A3 and A4) and CtBP2 (red, a marker of synaptic ribbons, A5 and A6) to evaluate synaptopathy (A5 and A6, merged). (A1,A3,A5) Hair cells (blue) and neurons (green) remain intact in young mice. (A2,A4,A6) Neuronal filament in auditory epithelium (green, A4) are notably destroyed in the aged mice. (B) Many of the IHC and OHC (blue, Hoechst) are significantly diminished in aged animals compared to young mice. © Number of presynaptic marker (CtBP2, red) per 10 hair cells was quantified. Aging mice had a significant decrease in CtBP2 counts as compared to the young, indicating significant loss of a marker of synaptic ribbons in aging animals. Scale bar = 30 μm. Graphs represent mean ± S.E.M. **** p < 0.0001. Unpaired t-test. n = 3.
3.2. Cochlear Microcirculation and Morphologies
Transmission electron microscopy (TEM) was conducted to investigate the morphological changes of the hair cells (Figure 4) and stria vascularis (Figure 5) in young (2 months) and aged (20 months) mice. Young inner and outer hair cells (2 months) showed a healthy appearance and normal mitochondrial morphology with well-defined cristae (Figure 4C,G). At 20 months of age, both IHCs (Figure 4B) and OHCs (Figure 4F) had numerous vacuoles in the cytoplasm. Aging mitochondria exhibited irregular sizes and electrolucent central matrices with undistinguishable, absent, and/or nonparallel disorganized cristae (Figure 4D,H). Ultrastructural analysis of cochlear lateral wall architecture revealed stria vascularis degeneration in aging cochleae, as indicated by numerous vacuoles and enlarged intercellular spaces (red arrows, Figure 5B) in all three layers of strial cells, marginal (Mc), intermediate (Ic), and basal (Bc) cells. Compared to young mice, aged stria vascularis exhibited damaged mitochondria with disorganized dysmorphic cristae (Figure 5D). To explore how aging changed cochlear microcirculation, blood flow was measured using laser Doppler flowmetry. Cochlear blood flow was significantly lower in aging mice than in young mice (Figure 5E, * p < 0.05, unpaired t-test). Thus, mitochondrial morphological changes in organ of Corti and lateral wall may contribute to the hearing loss and blood flow reduction found in ARHL.
.../...
T O A C C E S S T H E R E S T O F T H E S T U D Y, P L E A S E V I S I T T H E S O U R C E .
.















































