I would suggest a low chronic dosage for large rats, if to be administered on a daily basis. There is no need to consume huge amounts of oil on a daily basis. Possibly 1mL or less of oil per day as a chronic dosage would be sufficient - assuming 3 to 5mg of the original pristine fullerene per mL, depending on your body mass.
This seems reasonable to me. The original rat paper was a long term tox study, not a lifespan study. The effects were seen in a relatively short dosing regimen; i.e., the rats were dosed for a period of time then the dosing stopped, but they went on to live an abnormally long time. Unless there is a saturable elimination mechanism that requires a high dose in order to 'load' the system, I would expect any chronic dosing scheme to work reasonably well.
In the rat paper the C60 was mixed in the dark but I don't understand why this is so important. I could understand no exposing it to direct sunlight full of UV rays but what is wrong with ambient lighting?
Probably nothing. In papers like this, people try to cover all conceivable bases, because they know that everyone is going to nitpick the paper to death once it's published. The same sort of nonsense used to happen in resveratrol papers, where people treated it like it was highly unstable until someone finally published a paper showing that it was a very stable molecule.
Not the exact same animal, but does this give insight into the fate of our molecule.. is it integrated?
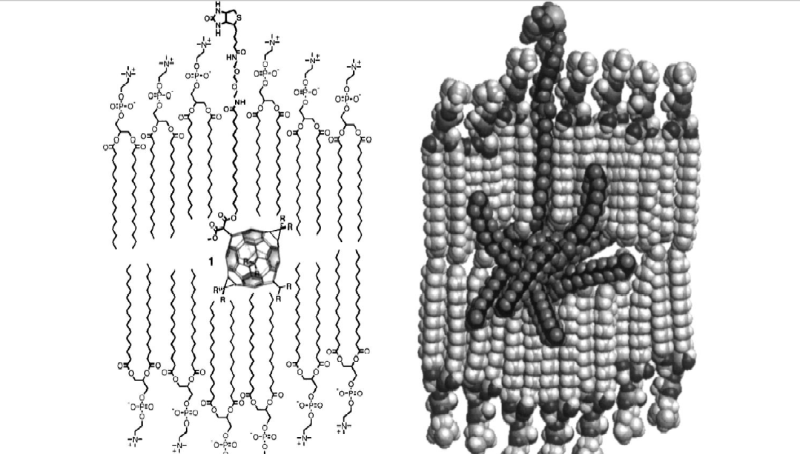
No, this isn't covalently attached to a biomolecule. This is a fullerene that has had long alkyl chains synthetically attached to it. One of the chains has a polar head group designed to fix the location of the fullerene in the membrane relative to the surrounding solution. I've seen the term 'lipofullerene' applied both to dendrimer-like alkane-studded fullerenes like the one you posted as well as to a simple solution of C60 in squalane. The use of the term for oil solutions of fullerenes is kind of marketing-speak, if you ask me. Chromatography of an olive oil-fullerene solution would show whether or not the fullerene is forming an adduct with any of the lipid molecules.
I'm pretty sure that isn't happening, and that it's just an ordinary solution. Edit: After more study, It looks like C60 does indeed form adducts with the fatty acids in olive oil under the conditions Baati used. I'm updating this in the event that people are reading these early posts. Blue text indicates edits.The question in my mind is: Do fullerenes react in some way with biomolecules so that they stay in the body permanently, and are not identified by typical analytical methods? The way that they extended life dramatically in the rat paper, even though the dosing stopped completely at a relatively early point in the rats' lives suggests something like this. I have a harder time imagining a mechanism whereby the rat biochemistry was 'reset' from a middle-aged state back to a youthful state, at which point normal aging proceeded, as though a time shift had occurred. At this point, however, I can't think of any obvious mechanisms for a fullerene to react with a biomolecule at body temperature, but I haven't researched the issue. Another possibility is a highly stable physical (non-chemical) interaction that keeps the fullerene located somewhere. Again, nothing is jumping out at me as a mechanism for such an interaction, but that doesn't mean it couldn't happen.
Edit: I now think that it is likely that fullerenes, either free or bound to a fatty acid, may react with another fatty acid in a biological membrane via a Diels-Alder mechanism.
Edited by niner, 28 June 2012 - 05:39 PM.
 2000px-Buckminsterfullerene.svg.png 267.36KB
0 downloadsWhen I suggested that round legospherane nanodiamonds the same size as longevity fullerenes could be hydrogenated be like fullerenes I was actually thinking that fullerenes had hydrogens on them. (silly) there is a different possibility that is even niftier some reactions create = links from - links, putting legosperoid nanodiamonds through those reactions would give the partially saturated linkages of fullerenes at the nanodiamond surface.
2000px-Buckminsterfullerene.svg.png 267.36KB
0 downloadsWhen I suggested that round legospherane nanodiamonds the same size as longevity fullerenes could be hydrogenated be like fullerenes I was actually thinking that fullerenes had hydrogens on them. (silly) there is a different possibility that is even niftier some reactions create = links from - links, putting legosperoid nanodiamonds through those reactions would give the partially saturated linkages of fullerenes at the nanodiamond surface.































 This topic is locked
This topic is locked