.
Posted yesterday by Phoebus here.
S O U R C E : International Journal of Biological Sciences.
Abstract
Changes in mitochondrial structure and function are mostly responsible for aging and age-related features. Whether healthy mitochondria could prevent aging is, however, unclear. Here we intravenously injected the mitochondria isolated from young mice into aged mice and investigated the mitotherapy on biochemistry metabolism and animal behaviors. The results showed that heterozygous mitochondrial DNA (mtDNA) of both aged and young mouse coexisted in tissues of aged mice after mitochondrial administration, and meanwhile, ATP content in tissues increased while reactive oxygen species (ROS) level reduced. Besides, the mitotherapy significantly improved cognitive and motor performance of aged mice. Our study, at the first report in aged animals, not only provides a useful approach to study mitochondrial function associated with aging, but also a new insight into anti-aging through mitotherapy.
Introduction
Mitochondrial dysfunction, including decreased oxidative phosphorylation capability and increased reactive oxygen species (ROS) production, is substantially responsible for aging and age-related features [1]. Studies in various organisms, such as nematodes, Drosophila, rodents, and humans, have strongly supported that aging is closely associated with mitochondrial dysfunction [2,3]. Thus, protection of the mitochondrial structure or stimulation of mitochondrial function is considered as practical ways in anti-aging [4,5]. However, since most of the mitochondrial damage is irreversible during aging process, the agents can always provide limited protection.
Mitochondrial therapy (mitotherapy) is to transfer functional exogenous mitochondria into mitochondria-defective cells for recovery of the cell viability and consequently, prevention of the disease progress. Accumulating evidence has indicated that exogenous mitochondria can directly enter animal tissue cells for disease therapy following local and intravenous administration [6,7]. In our recent reports, systemic injection of isolated mitochondria could reduce liver injury induced by acetaminophen and high-fat diet through improving hepatocyte energy supply and decreasing oxidative stress [8,9]. Therefore, we assumed that the mitochondria isolated from young animals (young mitochondria) into aged ones might play a role in anti-aging.
In this study, we intravenously administrated the young mitochondria into aged mice to evaluate whether energy production increase in aged tissues or age-related behaviors improved after the mitochondrial transplantation. The study, for the first time, offer new insight for mitotherapy on aged animals and give important evidence of understanding mitochondrial function in aging.
Results
Supplement of young mitochondria in aged mouse tissues after mitochondrial administration
The mitochondria were isolated from young mouse liver. Under the microscope, the mitochondria displayed spherical shape with good dispersion. Besides, double membrane structure and cristae of the isolated mitochondria remained intact, observing by TEM (Fig. 1A). It's well known that increasing age in mammals correlates with increased levels of mtDNA mutations and a deteriorating respiratory chain function, and aging-associated point mutations and deletions of mtDNA accumulate in a variety of tissues of aged animals, such as muscle, liver, lung, kidney, brain, heart, and spleen [10,11]. Among mtDNA mutations of aging, 4236 bp deletion of mtDNA from nt8884 to nt13120 bp is suggested as one of the most common deletions, and the deletion proportion to wild-type mtDNA is intensely correlative with aging [12].
Here, the supplement of young mitochondria in aged mouse tissues was measured by deleted mtDNA ratio. Tissue mtDNA was respectively extracted after mitochondrial administration for competitive PCR reaction by using primers F1, F2, and R simultaneously. Under UV, almost all PCR products showed two bands that appeared at 469 bp and 256 bp (Fig. 1B), in which the 469 bp band represents as wild-type mtDNA, while the 256 bp band as deleted mtDNA. The images showed that 256 bp band was weak in tissues of young mice, while high photodensity exhibited in the aged mice (Fig. 1B). The relative content of deleted mtDNA significantly increased in aged mouse tissues compared with that of young mice (Fig. 1C). However, the photodensity of 256 bp band reduced (Fig. 1B), and the ratio of deletion and wild- type mtDNA decreased in aged tissues after the mice repeatedly received mitochondrial administration (Fig. 1C).
Intracellular mitochondrial heteroplasmy after mitochondrial administration
Tissues with high metabolism are particularly vulnerable to mitochondrial dysfunction. Encephalopathy and myopathy are common phenotypes in mitochondrial disorders. Here we used TEM to observe mitochondrial morphology of the brain and skeletal muscle, meanwhile, the mitochondria activity was measured. Brain mitochondria in young mice showed intact and parallel cristae, while mitochondria in aged mice exhibited vacuoles cavitation, shrinkage, and reduction of mitochondrial cristae (Fig. 2A). Along with the changes of mitochondrial structure, the mitochondrial activity decreased significantly (Fig. 2B). However, mitochondrial activity increased in mitochondria-treated aged mice, and the mitochondria exhibited obvious heteroplasmy with the coexistence of intact and abnormal mitochondria (Fig. 2B). In addition, mitochondrial structure damaged and activity reduced in skeletal muscle of aged mice, while intact mitochondria appeared and mitochondrial activity increased after the mice received mitochondrial administration (Fig. 2A and 2B).
Effect of the mitotherapy on energy and redox production in the brain
Because the brain is sensitive to age-related mitochondrial impairments, here we examined the mitochondria-associated biochemical properties of the brain after mitochondrial administration. Activities of mitochondrial key enzymes of aerobic oxidation, including pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, and NADH dehydrogenase, decreased in aged animal brains (Fig. 3A, 3B and 3C), and these decreases were consistent with the loss of mitochondrial bioenergy production (Fig. 3D). However, mitotherapy partly recovered the enzyme activities and supplemented mitochondrial function in the energy supply of aged mice (Fig. 3A-D).
Mitochondria are not only the primary producers of energy in cells but also the main source of ROS. It has been known for a long time that dysfunctional mitochondria in aged animals are more prone to produce excessive ROS and leak it into the cytosol [13], as the result of an accumulation of damage to biomolecules causing cell injury and subsequent death. In the study, levels of ROS and MDA (peroxidation product of lipid) increased in aged mouse brain (Fig. 3E and 3F), along with a decrease of anti-oxidant GSH (Fig. 3G). However, the mitotherapy reduced ROS and MDA levels, probably because young mitochondria contain various antioxidants and can diminish excessive ROS.
Mitotherapy improved learning and memory ability of aged mice
To evaluate whether mitotherapy improve learning and memory ability of brain, the water maze task was used. In the navigation test, swimming latency of aged mice was longer than that of young mice from the second session, while the latency significantly decreased after mitochondrial administration (Fig. 4A). Nevertheless, there was significant difference between young mice and mitochondria-treated aged mice from the second session, suggesting that young mitochondria could improve the learning and memory ability of aged mice but cannot completely reverse the cognitive ability to the level of young mice. Meanwhile, swimming speed in the water maze task was determined. The results showed that aged mice had the slowest velocity in the three groups, and mitotherapy increased the swimming speed of aged mice (Fig. 4B), although the mice did not swim as fast as the young control. In probe test, both time spent and distance swam in target quadrant of mitochondria-treated aged mice were remarkably longer than that of aged mice (Fig. 4C, 4D and 4E), indicating that mitotherapy improved learning and memory function of aged mice.
Enhanced skeletal muscle function after mitotherapy
Hypofunction of skeletal muscle is one of the most presenting features of aging. Loss of muscle strength with age is closely associated with energy deficiency [14]. Under TEM, broken mitochondria showed in the skeletal muscles of aged mice, and residual mitochondria exhibited vacuoles and cristae abnormalitie (Fig. 2). Enzyme activities of mitochondrial pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, and NADH dehydrogenase, significantly decreased in skeletal muscles of aged mice (Fig. 5A, 5B and 5C), and correspondingly, ATP level decreased (Fig. 5D). Moreover, ROS and MDA levels in skeletal muscles of aged mice increased while GSH content reduced. However, administration of young mitochondria reversed the changes of energy deficiency and redox condition, implying that the sport organ would have higher energy supply than aged control in physical endurance tests.
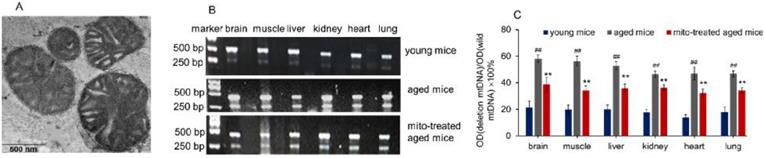
Figure 1
Supplementation mitochondria into tissues of aged mice. (A), the injected mitochondria were observed under TEM. (B), competitive PCR to detect the deleted mtDNA and mutant mtDNA. ©, quantitative PCR to quantify the ratio of deleted mtDNA to mutant mtDNA in brain, skeletal muscle, liver, kidney, heart, lung of aged mice. All data were expressed as the mean ± SEM. Mito, mitochondria. ## p < 0.01 compared with the ratio of the young mice, and **p < 0.01 compared with that of the aged mice. Student's t test was employed to compare the difference between the groups. Three independent replicates were used for each tissue.
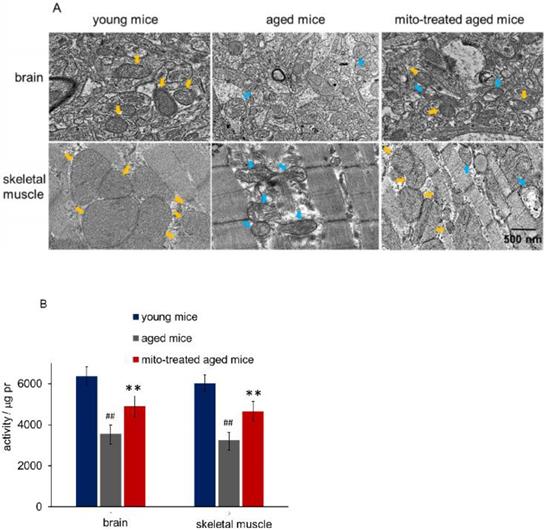
Figure 2
Mitochondria in brain and skeletal muscle. (A), the representative images of tissue mitochondria under TEM. The intracellular healthy mitochondrial numbers increased after mitochondrial transplantation. Yellow arrows point to healthy mitochondria, while blue arrows to aged mitochondria. (B), activities of isolated mitochondria in brain or skeletal muscle. Mito, mitochondria. The difference was analyzed by Student's t test. ## p < 0.01 compared to the young mice, and **p < 0.01 with the aged mice.
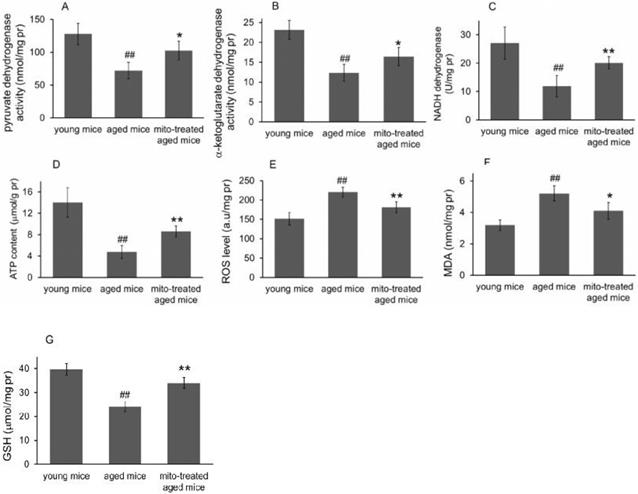
Figure 3
Effects of mitochondrial transplantation on bioenergy and bioredox of mouse brians. The injected mitochondria were isolated from the young mice. Activities of pyruvate dehydrogenase (A), α-ketoglutarate dehydrogenase (B), and NADH dehydrogenase © were respectively measured. (D), ATP content. (E), ROS level. (F) MDA content. (G), GSH content. The data were expressed as mean ± S.E.M (n = 4 for each group). The difference was analyzed by Student's t test. ## p < 0.01 compared to young control, and *p < 0.05, **p < 0.01 with aged group.
Forced swimming test is one of the most commonly used animal models for evaluation of muscle endurance. The mice treated with the mitochondria showed significantly longer swimming time relative to the control mice, from 7.2 ± 2.4 s to 13.3 ± 3.2 s, suggesting that mitotherapy could increase mouse sport endurance (Fig. 6A).
Then the mice were tested in the rotarod test. The aged mice showed a significant decrease from the second session of the latent period, relative to the control mice (Fig. 6B). However, the mice treated with the mitochondria showed an increase in the latent period from the second session, although the muscle tolerance of the mice was not as good as that of the young mice. These data suggest that the mitochondria could improve sport performance of aged animals.
Mitotherapy increased phagocytosis of macrophages
Besides cognitive and motor performance, ATP-dependent immune responses of macrophages reduce in aged animals [15,16], which are particularly at risk for developing chronic diseases. We have identified that exogenous mitochondria are not able to enter red blood cells because the cells lack endocytic function [17]. Here CD45 immunofluorescence staining was performed to examine whether the mitochondria could play roles in leukocytes (CD45-positive cells). The results showed that fluorescence-labeled mitochondria could arrive in CD45+ cells, including macrophages (Fig. 7A). Therefore, we further determined the effect of mitotherapy on phagocytosis of macrophages.
Carbon particle clearance test is a common used method to examine phagocytosis function of blood macrophages. Clearance index and phagocytic index significantly decreased in the aged mice, while the mitotherapy partly restored the phagocytic ability of macrophages in aged mice (Fig. 7B and 7C), while no significant changes were observed in the liver and spleen index (Fig. 7D). The results suggested that mitotherapy could stimulate macrophage activation, leading to enhance cellular immunity to exogenous particles.
Discussion
Mitochondria are cells' powerhouse that is indispensable for energy production and cell survival. One of the most highly investigated theories of aging is the mitochondrial theory of aging [18], which contains a central principle that decreased mitochondrial function is closely associated with aging. Here, we suggest that mitotherapy can supplement bioenergy and reduce the oxidative stress, as well as decrease the ratio of deletion and wild-type mtDNA, leading to improvement of cognitive and motor performance in aged mice. Intriguingly, the mitotherapy can activate the phagocytic activity of macrophages. The reversal effect of the mitochondria on aging provides new evidence to support the mitochondrial theory and opens a novel avenue for anti-aging.
Accumulation of mtDNA mutations would deteriorate respiratory chain function that underlies cell and tissue aging. It is well known that mtDNA encodes 37 genes, including 22 tRNA, 2 rRNA, and 13 protein components of oxidative phosphorylation. Differently from nuclear DNA, the mtDNA lacks damage-repair mechanisms, and the mutations are susceptibility to accumulate. A recent study identified that the onset of aging symptoms would be determined by the ratio of mutant to wild-type mtDNA, with a typical threshold effect [19], and thus the mice with a high mutant ratio of mtDNA exhibit advanced aging phenotypes. Although aging-related mtDNA mutation is regarded as incurable and has to wait for the development of genome editing techniques, the supplement of healthy mitochondria will have great promise to decrease the mutant ratio of mtDNA and then slow down the aging phenotypes.
The mitochondrial theory of aging holds that a decrease of ATP supply and an increase in ROS level cause the damages of cell components, resulting in senescence [20,21]. Cellular energy is mainly produced through oxidative phosphorylation taking place within mitochondria. Mitochondria in young animals can produce sufficient energy along with eliminating successive ROS. However, mitochondria in aged animals are characterized by reduced oxidative phosphorylation, increased ROS level, and diminished anti-oxidant defense. Much of the evidence have suggested the ROS produced by mitochondria damage cellular component and induce cell injury, which subsequently causes aging and death [22,23]. Therefore, scavenging ROS with antioxidants was recognized as an effective strategy to prevent aging and age-related diseases. However, increasing studies reveal that antioxidants do not reduce animal mortality [24,25], and some of them, such as β-carotene, vitamin E, and higher doses of vitamin A, can increase mortality, since low concentration of ROS caused by antioxidants inhibit ROS-mediated cellular signaling pathway [26]. Differently from the anti-oxidants, healthy mitochondria play an important role in maintaining ROS homeostasis through producing and scavenging ROS. Mitochondria coupling with the electron leak in respiratory chain produces ROS, meanwhile mitochondrial enzymatic and non-enzymatic anti-oxidant systems, such as superoxide dismutase and GSH, rapidly eliminate ROS [27,28]. In addition, accumulating evidence identify that the exogenous mitochondria could decrease ROS level and increase GSH content after mitochondrial administration, which could be the biochemical mechanisms of recovering cell function and restoring cell viability [7,29].
Here, we used an organ of central nervous system (brain) and a peripheral tissue (skeletal muscle) to study the effect of mitotherapy, since the most obviously and commonly recognized features of aging is energy decline in brain and skeletal muscle function that affects every aspect of human life, such as learning and memory, exercise, posture maintenance. In addition, the brain is a highly energy-consuming organ that requires about 20% of body oxygen with high oxidative phosphorylation to fulfill its function [30]. Thus, it is not surprising that mitochondrial dysfunction can cause disturbances in brain energy metabolisms, leading to the decreases in learning and memory ability (one common feature of aging and neurodegeneration diseases). In the previous study, we have suggested that mitotherapy could prevent Parkinson's disease in an experimental mouse model [17]. Besides, a current report confirms that exogenous mitochondria following intravenous administration can cross the blood-brain barrier (BBB) to treat depression in mice [31]. Although the mechanism of penetrating BBB of mitochondria remains unclear, it might be ascribed to a transcytosis pathway, which is a fundamental biological process used by macromolecules and particles to cross BBB [32]. The microvesicles containing mitochondrion may be formed during the transcytosis process. When the microvesicles enter the cells, the fusion of the vesicle membrane with the cytosol membrane would potentially make the intact mitochondria into the cytosol [33]. The entry mechanism of mitochondria into cells would be associated with actin-mediated endocytosis because actin polymerization inhibitors can prevent the internalization of mitochondria by cells [34,35]. After the mitochondria enter cells, they are transported to lysosomes, then majority of the mitochondria can escape from the lysosomes and play roles in cytosol [36]. Moreover, it has been identified that exogenous mitochondria can promote neurogenesis, and activate the expression of brain-derived neurotrophic factor (BDNF) in the reported articles [7,37,38]. Here we further identify the organelle therapy improve the cognitive and motor performance in aged animals, suggesting that the mitochondria could be used as a promising candidate to treat aging and age-related diseases.
.../...
.












































