Some thoughts on telomerase inhibition and the action of splicing regulators like resveratrol
Resveratrol and potentially other compounds that increase telomerase through splicing factors have far more effect on senescent cell cultures than those still passaging. Compare [1] to [2]. There is also a wealth of other literature on the more modest effects of resveratrol and normal cell cultures.
These substances have not been shown to increase telomerase at Sierra sciences (Bill Andrews) where they use a standard cell culture assay (various interviews).
They also only rejuvenate a subset of the cell culture [1] and [2].
This suggests to me that they are only rejuvenating cells rendered senescent by telomere erosion, not those with damaged DNA, etc. See the question at the end of this interview [3] for Faragher's position on this.
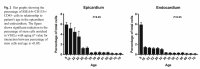
The fact that telomerase is boosted far more in cells already senescent (see also the effect of tocotrienols on cells at various stages [4] Fig 7d) also suggests such substances are operating via the short telomere effect - whereby short telomeres are preferentially elongated by telomerase because of the fact short telomeres tuck into a different location than long telomeres (See Shay and Wright work [5]), and therefore do not have the same degree of inhibition by the presence of the telomere. Note it is likely that telomerase is primarily inhibited by the telomere tail itself, which when long tucks into a location close (once the DNA is wound up) to HTERT, but even once this is removed other repressors are still in place. It is my opinion that splicing factors are this back up repression,making sure even if telomerase is produced it is in an inactive form with introns still present. It is this alternative splicing that is reversed by resveratrol until the telomere is long enough to re-extablish primary telomerase inhibition. This is most likely why telomerase upregulation by resveratrol is so short lived.
Another way of increasing telomerase is via unwinding DNA, with HDAC inhibitors (i.e. sulforaphane). This might be a way of elongating even long telomeres, by again removing the influence of the telomere on HTERT - it would still be tucked in a the same location but removed from HTERT but the unwinding of histones. Various papers have looked at combining different compounds and achieved synergistic effects [6].
Then there are substances like epitalon that are purported to increase telomerase by interpolating itself in-between the DNA strands at the HTERT location and force expression that way. The work was done in Russia [7], and hasn't been replicated elsewhere (so treat it with caution), but it is supposed to be able to match Henrietta Lacks cells for telomerase expression [8], and my results suggest it is effective.
There are concerns that increasing the lifespan of a cell line will increase their epigenetic age via methylation changes, possibly because the time such somatic cells exist before being replaced by the epigenetically younger stem cell pool will increase. There is evidence for this as when my telomere age decreased by 3 years using epitalon, my epigenetic age as measured by methylation patterns increased by a similar amount. It is an open question how much this matters. I've pointed out before that the increase in epigenetic methylation aging caused by telomere elongation is not the same as that experienced during normal aging. For example those on sartans [9] have been shown to have an older epigenetic methylation age. But the increase in epigenetic methylation age that comes with old age (and which is correlated with lower survival) is not occuring because telomeres are longer but because they are shorter. As stem cells lose telomeres and/or numbers, they slow down their replacement of the soma and hence those cells must survive for longer. So just as longer telomeres in somatic cells mean the soma CAN endure (i.e. self-renew) for longer without replacement, shorter telomeres (in stem cells) mean the soma HAS to survive for longer without replacement (as the replacement cells are in short supply). In the case of shorter telomeres the diagnosis is terminal. With longer telomeres a longer life beckons, unless the somatic cell line is kept going for so long it gets damaged in other ways. It is worth bearing that possibility in mind. I intend to continue testing both telomeres and epigenetic methylation age to keep a close eye on this. I am hoping it is possible to balance both. There is also the possibility in the future of boosting telomeres only in stem cells via TERC (the RNA template) rather than TERT (the protein) [10]. I've posted on this previously. That's enough for now.
[1]
https://www.ncbi.nlm...les/PMC5645932/
[2]
https://link.springe...522-020-09896-6
[3]
https://m.youtube.co...o&v=cBmrKaBlQ6M
[4]
https://www.hindawi....ri/2011/506171/
[5]
https://www.ncbi.nlm...les/PMC5826875/
[6]
https://pubmed.ncbi....h.gov/27433836/
[7]
https://www.ncbi.nlm...les/PMC7037223/
[8]
https://link.springe...A:1025493705728
[9]
https://www.ncbi.nlm...les/PMC6286862/
[10]
https://www.cell.com...87?showall=true