A new paper by Tony Wyss-Coray:
Molecular hallmarks of heterochronic parabiosis at single cell resolution
"We performed single-cell RNA-sequencing on 13 organs to reveal cell type specific responses to young or aged blood in heterochronic parabiosis. Adipose mesenchymal stromal cells, hematopoietic stem cells, hepatocytes, and endothelial cells from multiple tissues appear especially responsive. On the pathway level, young blood invokes novel gene sets in addition to reversing established ageing patterns, with the global rescue of genes encoding electron transport chain subunits pinpointing a prominent role of mitochondrial function in parabiosis-mediated rejuvenation. Intriguingly, we observed an almost universal loss of gene expression with age that is largely mimicked by parabiosis: aged blood reduces global gene expression, and young blood restores it. Altogether, these data lay the groundwork for a systemic understanding of the interplay between blood-borne factors and cellular integrity."
Comments on Hallmarks of heterochronic parabiosis
Aging (or old blood) affects the gene expression levels of different cells differently (they have different ‘Differentially Expressed Genes’ (DAG)), but there is a lot of agreement between the cell-specific DEGs for both aging and old blood. For some cells, the agreement is very high indeed (~0.6-0.84 for Endothelial Cells, Hematopoietic Stem Cells, Mesenchymal Stem Cells in Adipose Tissue) suggesting that these cells in particular primarily age extrinsically (i.e. via factors in the blood).
Note this does not mean other cells do not age intrinsically, of course.
Later on in the paper they talk about how some cells age in co-ordination with the tissue in which they reside, whereas other cell types ignore the tissue they’re in and instead age more in step with the tissue they come from (i.e. some bone marrow cells).
By contrast to the cell-type specific changes with age (or old blood), the rejuvenation provided by young blood seems to act on different cells in mostly the same way, via the mitochondrial pathways of the electron transport chain*.
*Weird exception that may prove the rule: brain endothelial cells (BECs) have been observed to undergo increased expression of electron transport chain genes with age. This effect is replicated by exposure to aged mouse plasma and reversed by exposure to young mouse plasma in vivo (ref: https://www.cell.com...81?showall=true Brain Endothelial Cells Are Exquisite Sensors of Age-Related Circulatory Cues).
My note: this suggests the solution to the general downregulation of the electron transport chain is not merely ETC upregulation, as this would be counterproductive to BECs, but instead a single or multiple corrections in the blood that either up- or down- regulates ETC function towards a youthful state as required through some indirect mechanism.
Some further thoughts on the problem: given we know the alteration of ETC function is via extrinsic factors in the blood, it would probably be a mistake to think mitochondria can be fixed via some mitochondrial specific molecule such as ALA or Q10. It makes more sense to assume there is some sort of problem in circulation such that mitochondria are not getting enough of something they need or are getting too much or something they do not need. So for example increasing circulation might give the mitochondria more of whatever it is they need that is present in the blood, or breaking down some sort of blood borne toxin might be the key to avoiding overloading the mitochondria with that toxic compound. This marks a significant change in the approach to reversing aging in that we are treating the blood and the circulation first and the cell second. It bears some similarity to the approach of Janic et al. in their short term statin-sartan treatment for pre-clinical atherosclerosis, discussed at length in this thread (for those that haven’t read it already it is one of the keystones of this thread: https://pubmed.ncbi.....gov/26214555/) .
One other thing that occurs to me from the paper when the Conboys replaced half the blood plasma with saline plus albumin and produced significant rejuvenation (https://www.ncbi.nlm...les/PMC7288913/), is that we mustn’t dismiss the importance of those blood proteins. For example, albumin, quite apart from its liver functions is an antioxidant buffer in the blood (https://pubmed.ncbi.nlm.nih.gov/21113488/), which should help mitochondria, particularly when in the procedure you are replacing partly oxidised with fully reduced albumin. Fibrinogen is an acknowledged 'damage associated molecular pattern protein' (DAMP) (https://journals.lww.com/shockjournal/Fulltext/2016/10000/Role_of_Elevated_Fibrinogen_in_Burn_Induced.7.aspx) and could also impede rejuvenation via decreased blood flow when it causes blockages in capillaries (that are not severe enough for a stroke). Transferrin moves iron, of which the main intracellular recipient is mitochondria, and intracellular transferrin is known to increase in Parkinson’s Disease (https://www.sciencedirect.com/science/article/pii/S1357272519300433), which also implicates it in mitochondrial dysfunction. Finally, immunoglobulins – the immune system is known to react to pieces of mitochondrial DNA in the blood (https://pubmed.ncbi.nlm.nih.gov/29125070/), leading to the rise in age related ‘sterile’ inflammation. At the very least these arguments suggest blood protein removal should be eliminated as a cause of aging in these parabiosis studies.
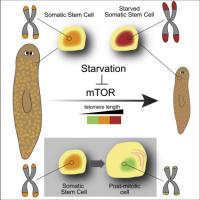
So where does this leave telomeres as a valid point of intervention in the aging process? This paper is particularly interesting as it shows how complex the age-related changes in genes are. Different genes are up or down regulated depending on tissue and cell-type. Much like the real situation with telomeres. At any given point there are cells with nice long telomeres and cells with short telomeres, sometimes living right alongside each other. Some tissues seem to maintain telomeres better than others. Even in a very old person there are cells with long telomeres that you can extract and passage in the lab. Sometimes a cell has long telomeres but is rendered functionally arrested because of mitochondrial damage. All these arguments have been used to dismiss telomeres as an important cause of aging. But the real picture is much more complex.
Now that we can see the body has a kind of aging imposed on much of it by systemic factors, it is possible to imagine that the machine just needs an oil change to make it as good as new. That would be wonderful. And indeed, this would not contradict the telomere story. How many times have I posted about ‘conditional reprogramming’ whereby old somatic cells with the right culture conditions would revert to a progenitor state and proliferate endlessly so long as the culture conditions were maintained? (for recap: https://linkinghub.e...2944013005944).Note that these culture conditions included telomerase. Perhaps young blood does something similar? Indeed, it is a central tenet of this thread that the statin-sartan treatment of Janic et al is doing just that. This is a throwback to the pre-Hayflick days when scientists though cells were immortal; it was just the body that aged. Perhaps they were not so wrong after all. With the right culture conditions.
Edited by QuestforLife, 17 November 2020 - 09:11 AM.