Hondo, go to 1fast400 and order there, for things they don't have check www.beyond-a-century.com, they are also reliable and cheap.
Hondo, if you care about your body and brain, shop at smi2le.biz. If you want to get something that "might be" or "could be" safe or what it is advertised as being, go to 1fast400 or beyond-a-century.com.
I am an independent consumer in this market. I have tested several of the products that are sold by smi2le.biz, in exchange for store credit. The products that are sold at 1fast400 or beyond-a-century.com are not tested by an independent third party; therefore you are making the gamble that these products are safe when imported from countries, such as China and India. If you feel comfortable taking large doses of substances from an unregualted market, that is your choice.
Click here and read this post about beyond-a-centurySource:
FDA WARNINGS ON CHINESE PATENT MEDICINESIA #66-10 - REVISED 2/21/91,
"Chinese Herbal Medicines",
ATTACHMENT A - 08/11/99, ATTACHMENT B - 6/6/00
PROBLEM : New drug without an approved new drug application (DRND) and labeling lacks adequate directions for intended use (DRDW). COUNTRY : HONG KONG (HK, 435) TAIWAN (TW, 281) PEOPLES' REPUBLIC OF CHINA (CN, 280) MANUFACTURER/ SHIPPER : MULTIPLE MANUFACTURER SHIPPER I.D.# : N/A IMPORTER'S I.D.# :
N/A CHARGE : When accompanied by drug claims, charge: "The article is subject to refusal of admission pursuant to 801(a)(3) in that it appears the labeling fails to bear adequate directions for use for its intended purposes (misbranded; 502(f)(1) and it appears to be a new dug without an effective new drug application [unapproved new drug; 505(a)]". When unaccompanied by drug claims, charge: "The article is subject to refusal of admission pursuant to 801(a)(3) in that it appears the labeling fails to bear adequate directions for use for its intended purposes [misbranded; 502(f)(1)]". RECOMMENDING OFFICE : LOS-DO
REASON FOR ALERT : Chinese herbal medications have a history, dating back to 1974, of containing strong prescription drugs. In 1974, four cases of agranulocytosis, resulting in the death of one person and extensive hospitalization of three others, were linked to use of these preparations. FDA analysis of pills involved in the illnesses found phenylbutazone and aminopyrine, and analyses of other "herbal medications" found methyl testosterone, prednisolone, diazepam, chlorzoxazone, and acetaminophen. In 1980, several illnesses and another death were linked to use of Chinese herbal medications, particularly chuifong toukuwan. Analysis of chuifong toukuwan from various sources found indomethacin, hydrochlorothiazide, chlordiazepoxide, lead, and cadmium.
In 1983, an additional brand of the chuifong toukuwan variety labeled only in Chinese (translated as product number 13 of attachment A below) appeared in the Portland, Oregon area. Trademark is a pair of concentric units surrounding a drag on intertwined about the numeral "7" (Seven Dragon Brand). The pills come 60 to a bag in a white plastic bag with a zip-lock closure. FDA analysis has found phenylbutazone at 10 to 18 milligrams per pill in the product.
In 1988, the Food and Drug Branch of the California State Department of Health Services issued a press release announcing an enforcement initiative against dangerous Chinese and other Asian ethnic medicines. Attachment B lists the products involved, the reputed manufacturer, and the hazardous ingredients contained. Currently, herbal pills are being marketed containing a combination of four drugs: diazepam, indomethacin, hydrochlorothiazide, and mefanamic acid. These pills are marketed under a variety of names and in a variety of packagings. They are spherically shaped, approximately 3/8" in diameter, and have a shiny black exterior. When the pills are cracked open, white crystalline material is observed to be imbedded in the brown interior.None of the products list the drug substances as ingredients. Investigation has shown that the pills originate from several sources, and usually enter the country via air mail shipments to health food stores, oriental food stores, novelty shops, and individual consumers. They are occasionally sold door-to- door.
These products are listed in attachment A. INSTRUCTIONS : Please inform your local U.S. Customs and Postal Service officials of our interest in these types of products, especially mail entries.
So the above is CLEAR EVIDENCE (proof) that Chinese imports which are sold in US with the presumption that they are free from contaminants (so that means that each and every one of the products listed above found to have adulterants were SUPPOSEDLY assayed for adulterants AND metals) ARE IN FACT DANGEROUS!
And note that the piracetam, aniracetam, pyritinol, L-Carnosine, and several more LE supps and nootropics ARE NOT subjected to the same scrutiny standards as these "PATENT" medicines. NOOTROPICS AND LE SUPPS ARE NOT REGULATED EITHER! So serious safety concerns might be associated with these nootropic/LE medicines!
Now: the PATENT medicines have a LOT more to lose by including adulterants; so presume what your bulk supplier has to lose by selling you deadly contaminants; and consider if that is a good exchange for your life.
Link to the source used belowToxic Heavy Metals and Undeclared Drugs in Asian Herbal Medicines
by Edzard Ernst
This article will also appear in Trends in Pharmacological Sciences. Issue 120
Abstract Asian herbal medicines are currently used by large sections of the population. Because they are not regulated as medicines and are freely available to everyone, serious safety concerns might be associated with these herbal medicines.
In this article, evidence suggesting that some Asian herbal medicines contain toxic heavy metals or undeclared prescription drugs is reviewed. In particular, Indian and Chinese preparations have been implicated.
Although adulteration with drugs is by definition fraudulent, the inclusion of heavy metals could be either intentional for alleged medicinal purposes or accidental.
Evidence from various countries implies that toxic heavy metals and undeclared prescription drugs in Asian herbal medicines might constitute a serious health problem. However, the majority of the data is anecdotal and insufficient to define prevalence figures.
Ways ought to be found to maximize consumer safety.
In most developed countries, Asian herbal medicines (AHMs) are becoming more and more popular [1]. However, usually AHMs are not regulated as medicines. Problems might arise as a result of the lack of adequate regulations, the pharmacological complexity of herbal products, and the paucity of information on the pharmacology and toxicity of these compounds. AHMs can be purchased from outlets ranging from health-food stores to Internet sites, and thus a crucial evaluation of their safety is relevant and important.
One obvious safety issue relates to the possibility that some AHMs contain heavy metals or undeclared drugs [2]. Based on a review of the recent medical literature (Medline, Embase 1990-2001), this article aims to summarize the recent evidence pertaining to this subject.
Indian Remedies Indian medical systems (e.g. Ayurveda and Unani) have a long and rich history of herbal medicine, and heavy metals have been a regular and deliberate constituent of traditional Indian remedies [3]. Thus, to use the term "contamination" with respect to the presence of heavy metals in such remedies might be misleading (see below).
A London-based toxicology unit published a case series of adverse events associated with traditional medicines that were reported to them between 1991 and 1995 [4]. Of 12 cases of poisoning with lead, arsenic or mercury, nine cases were associated with herbal remedies from India and the remainder was due to traditional Indian cosmetics (e.g., "surma.")
A recent exemplary case report from Italy [5] (box 1) exhibits many hallmarks of such cases: desperate parents, non-medically qualified healers, lack of product standards, undeclared ingredients, nondisclosure of usage and long-term medication, in addition to delay of diagnosis of poisoning and hence delay of effective therapy. Indian authors recently analyzed 31 Ayurvedic formulations obtained in India for their mercury content [6].
With the exception of one remedy, all exceeded the legal limits of 1 ppm mercury and 16 preparations exceeded the limits by more than two orders of magnitude. These authors also noted that huge variability of mercury content existed within one allegedly identical remedy manufactured by different companies.
No recent systematic investigations are available about the prevalence of heavy metal content of traditional Indian remedies on sale in developed countries.
Thus, a considerable degree of uncertainty continues to surround this area.
Chinese Remedies Numerous case reports and case series of heavy metal poisoning associated with the use of traditional Chinese medicines (TCMs) have been published [7]; lead has relatively often been implicated as the cause of such poisoning but mercury, cadmium, arsenic, copper, and thallium have also been found in TCMs [7].
Californian officials have screened for undeclared pharmaceuticals and heavy metals in imported Chinese remedies on sale in Californian herbal retail stores [8]. Seven percent of the 251 products tested contained undeclared pharmaceuticals (e.g., ephedrine, chlorpheniramine, methyltestosterone, and phenacetin). Twenty-four products contained at least 10 ppm lead, 36 contained an average of 14.6 ppm arsenic, 35 contained an average of 1,046 ppm mercury, and 23 had more than one contaminant and/or adulterant.
Koh and Woo [9] reported the detection of toxic heavy metals that exceeded Singapore's legal limits in 42 Chinese proprietary medicines. They collected 2,080 samples of such medicines in Singapore and tested them for heavy metal content. Forty-two different medicines were found to contain metals in amounts exceeding the legal limits.
Mercury was found in 28 products, lead in eight, arsenic in six, and copper in one. One product contained both mercury and lead and another product contained both mercury and arsenic. Melchart et al. [10] analyzed all 317 batches of dried Chinese herbs delivered to a German hospital of Chinese medicine.
Heavy metal content beyond the legal limits was detected in 3.5% of these samples. Obviously, heavy metals are not the only possible toxic ingredients in herbal remedies; contamination with herbicides, pesticides, microorganisms; or mycotoxins, insects, or undeclared herbal constituents are other relevant possibilities [2,11-13].
Moreover, contamination with toxic herbal constituents (e.g., through misidentification of the herbal ingredients) can be a serious problem. In Belgium, the use of a TCM contaminated with plants from the Aristolochia species resulted in an epidemic of subacute intestinal nephropathy. Many of the affected patients required kidney transplantation. When 19 kidneys and urethras removed from ten such patients were examined histologically, there were conclusive signs of neoplasms in 40% of cases [14].
Numerous case reports originating from countries such as Australia, Belgium, China, the Netherlands, New Zealand, United Kingdom, and United States demonstrate the adulteration of TCMs with synthetic drugs and associate the use of adultered remedies with health problems of the user [15]. The adulterants include a wide range of pharmaceuticals (box 2). The resulting clinical consequences are often serious and sometimes life threatening: agranulocytosis, Cushing's syndrome, coma, the excessive increase of the international normalized ratio (INR) have all been reported.
In other cases, the adulterants caused no symptoms at all and the problem was discovered only through routine check-ups or through the remarkably good clinical response, which turned out to be due not to the TCM but to the undeclared prescription drug.
Analyses are available of Chinese herbal medicines collected in Australia [16], Taiwan [17] and UK [18]. The largest of these studies is that of Huang and colleagues from Taiwan [17], who showed that 24% of all 2,609 samples collected contained at least one adulterant.
This high prevalence was due to the fact that the samples were associated with reports of adverse effects and poisoning, and possibly included low-grade folk remedies. Examples of recent case reports [19,20] are illustrated in boxes 3 and 4.
Concerns About the Safety of Asian Herbal Medicines
These data raise concerns about the safety of consumers using AHMs. Both toxic heavy metal content and adulteration with prescription drugs have been reported. To date, few data are available to calculate the prevalence of these problems reliably in developed countries.
A recent press release [21] of the British "Medicines Control Agency" stated that this regulatory body "continues to find potentially dangerous and illegal ingredients in TCMs. Recently TCMs have been found to include . . . mercury and arsenic . . . [and] prescription only steroids." It is notable that the majority of clinical problems occur with self-prescription of AHMs.
One could therefore argue that consulting an experienced herbal practitioner might avert adverse events; however, evidence is required to support this claim. Several possibilities exist to explain the presence of heavy metals in AHMs. First, heavy metals could be included intentionally for alleged medicinal properties.
Some Indian schools of medicine emphasize the importance of metals such as lead, copper, gold, iron, mercury, silver, tin and zinc for the proper function of the human body [22]. Ayurvedic textbooks, for example, take note of the toxicity of heavy metals and recommend special physicochemical processes that, according to ancient Indian belief, "detoxify" such toxic heavy metals (e.g. by heating them until they glow [23]).
In traditional Chinese medicine, mercury is part of some preparations under the terminology of "cinnabaris" (mercury sulfide), "calomel" (mercury chloride) or "hydrargyri oxydum rubrum" (mercury oxide). Such products are used for a variety of indications including, for example, as a tranquilliser, an anti-epileptic, for ulcers or to treat insomnia [9]. Lead is used as "Mi Tuo Seng" (Lithargyrum) [24] and arsenic as "Xiong Huang" (Realgar) [25] in the manufacture of several TCMs.
Strictly speaking, these constituents are thus not contaminants but ingredients deliberately included for a specific curative purpose.
Second, the presence of heavy metals might be the result of contamination during manufacture, for example, from grinding weights or lead-increasing containers or other manufacturing utensils [9]. Third, AHMs might contain heavy metals when grown on seriously polluted soil [26].
In this context it is relevant to note that TCMs might also contain animal and mineral products and that these too might be contaminated with heavy metals [27].
Although contamination can be accidental, adulteration is, by definition, fraudulent. The reasons why some AHMs contain prescription drugs are speculative. I suspect that some manufacturers include such ingredients to render their products more clinically effective. If this is the case, it seems obvious that the inclusion of prescription drugs is fraudulent and illegal.
Many consumers are motivated to try AHMs through a misconception that these remedies are inherently safe [28], and there is evidence that the (UK) daily press have their share in perpetuating this myth [29].
Approximately half of the individuals using herbal medicines do not tell their physician [30]. This level of non-communication further increases the risk to the consumer because doctors might fail to diagnose adverse effects caused by treatments of which they are not aware. The majority of people taking herbal remedies combine them with conventional drugs [30]. This opens the possibility of herb-drug interactions [31,32], which, in turn, further raises concern about consumer safety.
Recent evidence suggests that consumers are beginning to become concerned about the risks of under-regulation of dietary supplements, and the majority of US consumers now seem to support [33]: (1) the requirement that the Food and Drug Administration (FDA) review the safety of new dietary supplements before their sale; (2) increased authority to remove from sale those products shown to be unsafe; and (3) increased government regulation to ensure that advertising claims about the health benefits of dietary supplements are true.
How can the risk to patients be minimized? An appropriate strategy [34,35] (box 5) should follow several avenues. The consumer should be informed that "natural" does not necessarily mean 'free from risk' and that adverse effects as a result of AHMs are an undeniable reality.
Patients and physicians should be encouraged to talk about the use of AHMs and other complementary/alternative treatments [34] and the possibility of interactions of herbal medicines with prescribed drugs [31,32].
Regulators should consider measures to control this sector of healthcare more effectively. It is concluded that toxic herbal metals and undeclared drugs in AHM represent a potentially serious problem that puts consumers at risk. Means of minimizing this risk must be found and implemented.
Wang, Ang, b. 1615; Hu, Tsung-wen Shen-nung pen ts'ao pei yao i fang ho pien (Herbal and Prescriptions) China, 1740. 6 vols. from The National Library of Medicine.The New England Journal of Medicine -- September 17, 1998 -- Vol. 339, No. 12
To the Editor:
Asian patent medicines are one component of what are called traditional Chinese medicines. Asian patent medicines comprise multiple products, including herbs, plants, animal parts, and minerals, which are formulated into tablets, pills, or liquids for ease of use. They are widely available in herbal stores and have gained acceptance by the American public as a form of alternative medicine. However, many patent medicines manufactured in Asian countries contain toxic ingredients, such as heavy metals, as well as prescription drugs or unapproved ingredients that may or may not be identified on the label. (1,2) Some have caused serious illness in unsuspecting consumers. (3,4)
The California Department of Health Services, Food and Drug Branch, initiated a study to screen imported Asian patent medicines for undeclared pharmaceuticals and heavy-metal contamination, using gas chromatography-mass spectrometry and atomic-absorption methods. Our objectives were to establish a computer data base for these products; educate the public, the herbal industry, and the medical community about the potential danger of Asian patent medicines; and provide objective information about toxicity.
Of 260 Asian patent medicines that have been collected from California retail herbal stores, 14 had labels that declared pharmaceutical ingredients, and 3 had insufficient sample amounts. Of the remaining 243 products, 17 (7 percent) contained undeclared pharmaceuticals. The most common undeclared ingredients were ephedrine, chlorpheniramine, methyltestosterone, and phenacetin.
A total of 251 products were analyzed for lead, arsenic, and mercury; 9 other samples, including the 3 noted above, were insufficient for this analysis. Twenty-four products contained lead in a quantity of at least 10 parts per million (ppm) (range, 10 to 319; median, 29.8; mean, 54.9). Thirty-six products contained arsenic (range, 20.4 to 114,000 ppm; median, 180.5; mean, 14,553). Thirty-five products contained mercury (range, 22.4 to 5070 ppm; median, 329; mean, 1046); 2 of the 35 had labels that identified only pharmaceutical ingredients. The United States Pharmacopoeia limits heavy metals in most oral pharmaceuticals to 30 ppm, with lower limits for lead, arsenic, and mercury.
Of the 260 products we investigated, at least 83 (32 percent) contained undeclared pharmaceuticals or heavy metals, and 23 had more than one adulterant. The remaining products, which contained no detectable adulterants, cannot be assumed to be safe and free of toxic ingredients, in view of their batch-to-batch inconsistency, as well as limitations in our detection methods.
Richard J. Ko, Pharm.D., Ph.D.
California Department of Health Services
Sacramento, CA 94234-7320
Link to above article
BE CAREFUL!
And this post too about 1fast400
And consider the results of the assays I have conducted from products from smi2le.biz
Please consider the results of the assays of products from smi2le.biz after considering these definitions:
USP <231> Heavy Metals
The melting point is used in chemistry as a rough guide to purity, since a pure crystalline compound will generally melt over a range of a few degrees, and is depressed by impurities (or polymorphs). Liquefaction, and in many cases subsequent recrystallisation upon cooling follows melting. In the case of RLA, the material actually polymerizes below its melting point and you see no changes upon heating all the way up to 250 deg C, where the material begins burning. The only way to get a usable melting point is to have a device pre-set to 49 deg C, place a sample on a microscope cover slip and to drop it on the pre-heated surface. Even then you get polymerization in many cases rather than a clean melt even though the material is pure by HPLC. This is why we don’t use the melting point has the sole criteria of purity.
Click here for source
1. Smi2le.biz's idebenone; test conducted for melting point (54-55); moisture/loss on drying (0.519); satisfies USP specification for heavy metals at <0.05 parts per million (so the sample satisfies USP specification at less than 10 parts per million of lead, mercury, bismuth, arsenic, antimony, tin, cadmium, silver, copper, and molybdenum);

2. Aniracetam from smi2le.biz; test conducted for purity (98.11% pure; but don't forget to add the moisture level as well as moisture/loss on drying to the sample's integrity level);
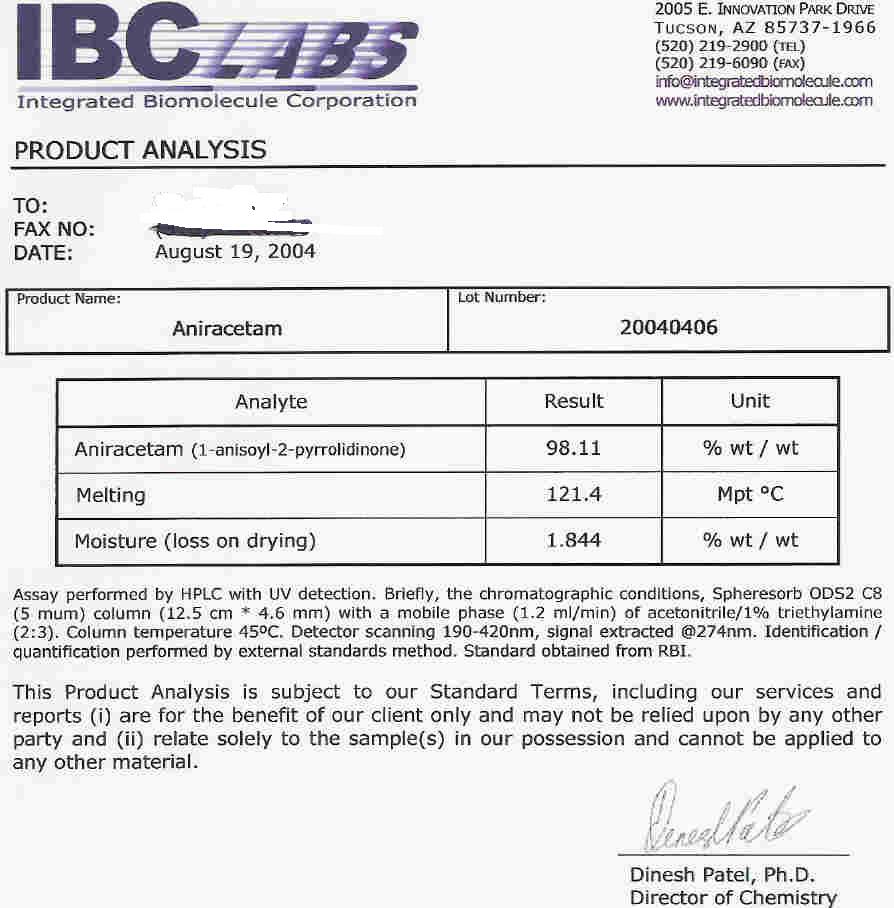
3. L-carnosine from Smi2le.biz; tested for melting point at 250 degrees celcius (which enables us to identify the substance by measuring the temperature at which a supplement dissolves) as well as heavy metals (satisfies USP specification at less than 10 parts per million of lead, mercury, bismuth, arsenic, antimony, tin, cadmium, silver, copper, and molybdenum);

To read more about the benefits of L-carnosine supplementation: click here
4. Oxiracetam from Smi2le.biz tested for tested for melting point tested at 168 degrees celcius (which enables us to identify the substance by measuring the temperature at which a supplement dissolves) as well as USP metal assay (satisfies USP specification at less than 10 parts per million of lead, mercury, bismuth, arsenic, antimony, tin, cadmium, silver, copper, and molybdenum);

To learn more about oxiracetam and its benefits, Click here.
5. Pyritinol from smi2le.biz tested for melting point to 138 degrees celcius (which enables us to identify the substance by measuring the temperature at which a supplement dissolves) as well as heavy metals: lead, mercury, bismuth, arsenic, antimony, tin, cadmium, silver, copper, and molybdenum):

To read more about the benefits, suggested dosages and the respective medical abstracts, simply click here.
To read earlier correspondences: click here and here






























 This topic is locked
This topic is locked



