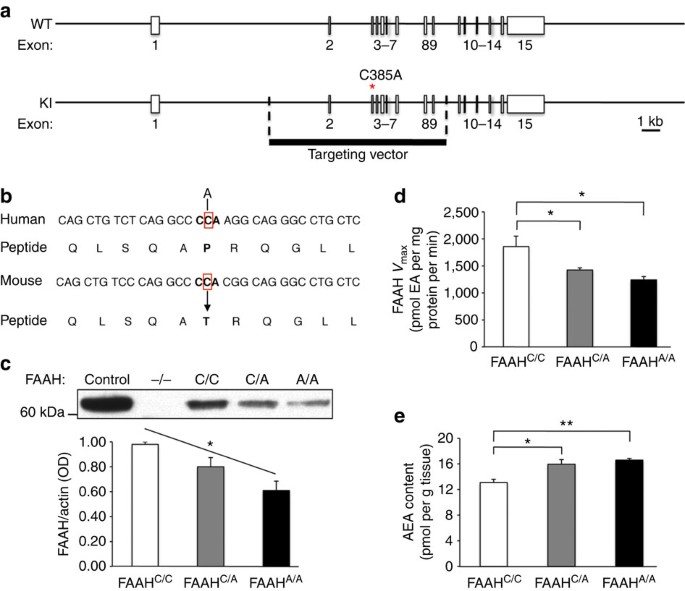
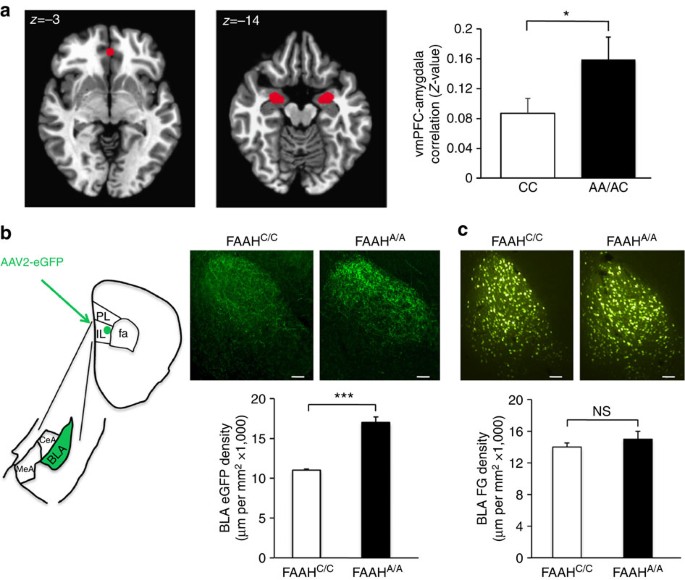
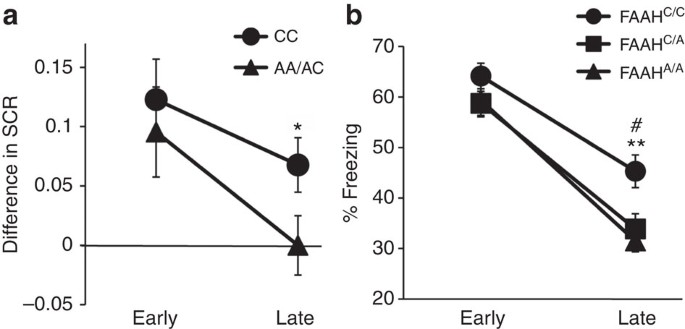
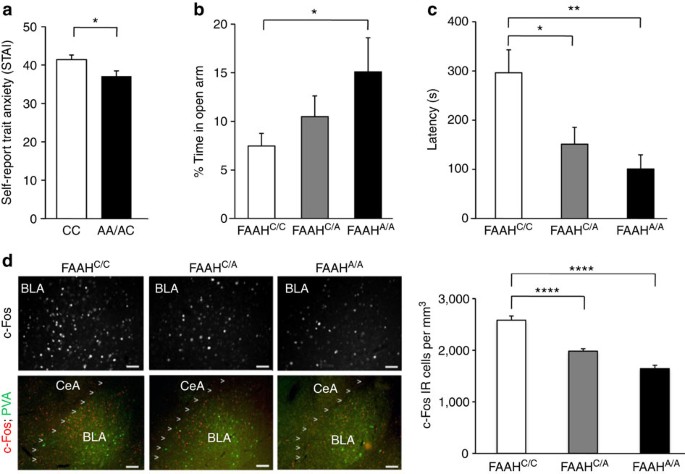
Frequency specific microcurrent in three wounded warriors
https://frequencyspe...ed-Warriors.pdf
MEDICAL ACUPUNCTURE
Volume 31, Number 3, 2019 # Mary Ann Liebert, Inc. DOI: 10.1089/acu.2019.1366
Frequency-Specific Microcurrent as Adjunctive Therapy for Three Wounded Warriors
Stephen J. Sharp, MD, MS, Mylene T. Huynh, MD, MPH, and Rosemarie Filart, MD, MPH
ABSTRACT
Background: Acupuncture is frequently offered for wounded warriors as a component of an integrated approach to pain and associated symptoms, with increasing availability at military treatment facilities and Veterans Administration hospitals. While medications can be effective for many patients, acupuncture and microcurrent therapies address the growing need to offer nonopiate, nonpharmaceutical therapeutics in inte- grative pain management. Frequency-specific microcurrent (FSM) is a newer, adjustable, microcurrent, elec- trical stimulation modality with applications for pain and other associated symptoms. Using low amperage, electrical current delivered transcutaneously affects and repairs tissues at the cellular level. Additionally, concomitant treatment with acupuncture is possible, which is particularly helpful when space and time limit the frequency with which acupuncture treatments can be provided.
Cases: For 3 wounded warriors, FSM was combined with acupuncture treatments, resulting in more-rapid reduction of their pain and associated symptoms; including memory problems, mental sluggishness, and post- traumatic stress disorder.
Results: FSM was found to be a safe, nonpainful, noninvasive treatment that could be administered concur- rently and beneficially with acupuncture.
Conclusions: While additional, more-rigorous studies are needed, this case series demonstrates the potential that FSM has within an integrated pain treatment program for wounded warriors.
Keywords: military acupuncture, energy medicine, frequency-specific microcurrent, wounded warrior
INTRODUCTION Acupuncture is an increasingly important part of
an integrated approach in the treatment of pain in the U.S. military wounded-warrior population. Acupuncture is offered at an expanding number of military treatment fa- cilities (MTFs) and Veterans Administration (VA) hospi- tals.1 However, many MTF and VA patients have additional symptoms and complex pain presentations. Therefore, in addition to medications, interventional procedures, and sur- gical options, a wounded-warrior integrated pain-management program may also include a combination of techniques, such
as acupuncture, electrical stimulation, yoga, mind–body ex- ercises, chiropractic care, rehabilitation therapies, behavioral health management, preventative and therapeutic physical
2,3 activities, nutrition, and lifestyle modifications evaluations.
While acupuncture alone is helpful for many patients, its op- timal use often requires a frequency of treatments exceeding the ability of many providers and facilities to accommodate.
Frequency-specific microcurrent (FSM) treatment is performed with a microcurrent device that is unique in its adjustability to different tissues, using low-amperage fre- quencies that can modulate pain as well as other associated symptoms.4–7 FSM treatment is based on the biology of
Pain Clinic, Department of Anesthesia, Walter Reed National Military Medical Center. Bethesda, MD.
The views expressed are the private views of the authors and do not reflect the official policy or position of the U.S. government, the U.S. Department of Defense or the United States Military.
189
Downloaded by Walter Reed NMMC (National Military Medical Center) from www.liebertpub.com at 08/07/19. For personal use only.
190
SHARP ET AL.
resonance and electrical signaling of cells.7–11 Unfortunately, there is limited clinical research on FSM to date, especially in conjunction with other integrative therapies such as acu- puncture. This article presents 3 cases in which FSM was applied concurrently with acupuncture and was found to re- duce symptoms, resulting in fewer visits, compared to acu- puncture alone. This case series also demonstrates the range of conditions for which FSM may be a safe, valuable ad- junctive therapy.
CASES
Case 1
A 40-year-old active duty physician presented with pro- gressive back, hip, and leg pain that was interfering with her regular physical activity and ability to take required physical- assessment tests. For example, running was very painful and, therefore, she had activity limitations. This patient reported that, although acupuncture treatments were helpful, she continued to have problems with returning to full regular exercise. FSM was added into her acupuncture sessions, using an FSM myofascial, trigger-point (MFTP) program.
After 2 combined FSM and acupuncture treatments, she began to run again without significant pain for the first time in several years. With additional treatments, she noted an effect like ‘‘peeling an onion,’’ wherein previous known areas of pain and injury improved in a reverse order from the date of the trauma. Although she had reductions in her pain, she ad- ditionally reported having mental sluggishness and difficulty with memory that seemed to relate to receiving the anthrax vaccine many years prior. For those symptoms, FSM was applied alternating between the FSM Concussion and FSM Brain Fog programs. After 2 treatments, she reported an im- provement and then, after an additional 2–3 treatments, she reported her memory had returned to her baseline pre-vaccine state. Occasional subsequent pain flares were addressed with further combinations of FSM and acupuncture.
Case 2
A 35-year-old male presented to the pain clinic with symptoms stemming from several deployments, with mul- tiple hard landings as a paratrooper, concussion from an improvised explosive device blast, and C-5–C-6 dislocation and fracture without neurologic sequelae. He reported multiple areas of pain, especially in his back and neck, migraine headaches, thinking and memory problems, and other post-traumatic stress disorder (PTSD) symptoms. Due to this patient’s complex symptom presentation, he was started on a combination of FSM and acupuncture as an initial integrated pain approach. At the first treatment, the FSM Concussion program was applied along with acu- puncture for his neck and back pain.
His chronic headache resolved almost completely after that first treatment. After the second treatment, his neck and back began to improve, and a second FSM program, for PTSD, was initiated. This resulted in the patient reporting clearer thinking and improved memory for 3–5 days. Con- tinued treatments resulted in similar improvements of lon- ger durations and increasing effectiveness. The subsequent FSM treatments included 1 or 2 of the Concussion, PTSD, and Brain Fog programs running concurrently or serially per session. He subsequently underwent a cervical spinal fusion and was lost to follow-up when he was transferred to a different geographic location for inpatient rehabilitation.
Case 3
A 32-year-old male presented to the pain clinic with symptoms of diffuse areas of pain including his neck, back, and abdomen. He also reported having mental sluggishness and a brain-fog feeling. He disclosed that he self-administered multiple supplements in the past, including several that were reported to have hormone-like effects, which were postulated as contributing to his symptoms. Following initial treatments with acupuncture, he reported improvements in pain reduc- tion. However, his mental sluggishness and brain-fog symp- toms persisted. Consequently, he received FSM in addition to acupuncture treatments in a dual FSM combination of the Concussion and Brain Fog programs.
After only a couple of sessions, this patient reported further reductions in his pain, mental sluggishness, and brain-fog symptoms. However, while he reported continued symptom reduction, his ongoing chronic severe behavioral- health issues complicated his course. Eventually, he re- quired transfer to another facility for behavioral-health management, halting this MTF’s integrated pain program for him; this patient was then lost to follow-up.
RESULTS
FSM resulted in reduction of pain as well as resolving brain fog, memory, and headaches in these 3 wounded warriors.
DISCUSSION
FSM is a newer, adjustable, microcurrent, electrical stimulation modality with applications for pain and other as- sociated symptoms. FSM is applied through an electronic de- vice like a transcutaneous electrical nerve stimulation (TENS) unit and falls under the U.S. Food and Drug Administration category of TENS devices. Yet, unlike TENS, FSM delivers a nondiscernable microcurrent that is not intended to trigger muscle contractions or pain. More importantly, FSM delivers a frequency that is tailored to specific tissues, as well as the injury/disorders specific to those tissue.4–7 While an
Downloaded by Walter Reed NMMC (National Military Medical Center) from www.liebertpub.com at 08/07/19. For personal use only.
FSM AND ACUPUNCTURE
191
indepth discussion of how FSM works is beyond the scope of this article, quantum physics has taught scientists that like at- oms, molecules and tissues vibrate at determined resonant frequencies.9,10 When a molecule/cell/tissue is sick or injured, there is a change from its basic resonant frequency.9,10 If the frequency is reset to the baseline resonant frequency, the cells and tissues heal more easily and more quickly.4–7,9–11
Like acupuncture, FSM can be tailored to the specific conditions being treated, thereby providing an individual- centered treatment. In this case series, several programs were applied for a range of pain-associated symptoms. The preinstalled MFTP program was designed for a broad range of muscle- and fascia-related problems. Other, more- specific pain FSM programs are often needed when pain stems from bone, ligament, or nerve injuries, but the MTFP program often provides a reasonable starting point. Given that many chronic-pain issues are now postulated to be fascial-related, the MFTP program was the initial program used for these 3 patients’ treatments.
Additionally, because it is now widely believed that acu- puncture channels travel in fascial or extracellular-matrix lay- ers, the present authors hypothesized that these treatments provided a synergistic effect between acupuncture and FSM. The second program applied was the Concussion program. While this program is useful for concussions/mild traumatic brain injury, as the name suggests, FSM developers have re- ported its additional usefulness for addressing emotional, psy- chologic, and/or spiritual—as well as physical—traumata. It appears to function as a ‘‘brain reset’’ method that is helpful for patients with a wide range of pain issues and other conditions.
The third program mentioned was the Brain Fog protocol, which targets the memory and attention issues that are fre- quently seen in wounded-warrior patients—again, stem- ming from physical and/or emotional causes. The PTSD program was applied in the second case. While anecdotal evidence has reported efficacy of the PTSD program, its 2- hour duration creates a clinic flow- and time-efficiency challenge, making it impractical for most routine clinic sessions. A shorter version of the program is being devel- oped to address the efficiency challenge.
Musculoskeletal pain stemming from a variety of causes is seen frequently—whether due to an acute in-theater in- jury; wear-and-tear injuries such as occurs in paratroopers or from walking around the desert with a backpack; ubiq- uitous sports injuries; or the most-common ‘‘I don’t know what caused it’’ injury. The ability to add a peeling-the- onion effect—that is, to delineate the pain sources better—is an added benefit of FSM as an adjunct to acupuncture treatment. Recent studies suggest that recurring injuries to the fascia layer could predispose patients to chronic-pain problems. Thus, it is important that the ability of FSM to help isolate and repair chronic fascial injuries appears to aid in long-term integrated pain treatment.4–7
The mental sluggishness and memory deficits related to the anthrax vaccine are not commonly reported problems
and, consequently, studies regarding its treatment are lack- ing. While a single case remains anecdotal, the potential of FSM to add a treatment benefit in a patient with symptoms related to the anthrax vaccine deserves consideration when treating any patient who might have this problem. This is especially significant given that there has been no reports of FSM increasing the risk of adverse effects in these patients. Moreover, another benefit of FSM treatment is its easy and safe application for the behavioral health and neurologic issues often associated with pain. The FSM Brain Fog program seemed the most relevant in this case, with the Concussion program as an optional alternative.
The second case was complicated by PTSD and traumatic brain injury in addition to the patient’s physical injuries. The third case also involved PTSD, but this was not directly related to a physical injury or concussion. To address the practical clinic-flow efficiency challenge, the FSM Con- cussion and Brain Fog programs, which run for 25 minutes each, provide the ease-of-fit within the acupuncture clinic session time-frame, which, in this MTF facility, is typically 30 minutes. In these 2 cases, the use of FSM treatment with the Concussion/reset program followed by alternating treatments with other indicated FSM programs, appeared to accelerate the recovery process.
The present authors acknowledge limitations in delin- eating the specific effect of any given FSM treatment in this case series. One limitation was that the patients re- ceived treatments within the rubric of an integrated, mul- tidisciplinary, multimodal pain program, thereby making it difficult to separate the effect of one treatment from the integrated program. A second limitation was that 2 patients (cases 2 and 3) had received care within an integrated pain program for a variable time frame before receiving FSM treatments. A third limitation is the number of clinic visits with FSM treatments were limited by the patients being discharged from care and lost to follow-up. Nevertheless, even with these limitations, the treated wounded warriors reported that, with the addition of FSM, they had noticeable changes during those sessions.
CONCLUSIONS
At present, while FSM has potentially wide applications for addressing a range of pain disorders and related condi- tions, with increasing clinical usage, current literature on clinical experience has been mostly case reports, such as with this case series.7–10 Some randomized controlled trials are planned or in progress; however, much more is needed. From this small case series, the present authors conclude that there is an added treatment benefit of symptom reduc- tion following the combination of FSM and acupuncture treatment for wounded warriors, to address their pain, PTSD, and other associated conditions. It is hoped these case reports will provide a stimulus for further research
Downloaded by Walter Reed NMMC (National Military Medical Center) from www.liebertpub.com at 08/07/19. For personal use only.
192
SHARP ET AL.
using FSM as an adjunct to acupuncture treatment and as part of an integrated pain-management program for woun- ded warriors.
ACKNOWLEDGMENTS
Special thanks to Carolyn McMakin, DC, who provided the impetus for us to learn FSM and to publish these cases.
AUTHOR DISCLOSURE STATEMENT
No competing financial interests exist.
REFERENCES
1. Walker PH, Pock A, Ling CG, Kwon KN, Vaughn M. Bat- tlefield Acupuncture: Opening the door for acupuncture in Department of Defense/Veteran’s Administration health care. Nurs Outlook. 2016;64(5):491–498.
2. HermanPM,SorberoME,Sims-ColumbiaAC.Complementary and alternative medicine services in the military health system. J Altern Complement Med. 2017;23(11):837–843.
3. Ross EM, Darracq MA. Complementary and alternative medicine practices in military personnel and families pre- senting to a military emergency department. Mil Med. 2015; 180(3):350–354.
4. McMakin CR. Oschman JL. Visceral and somatic disorders: Tissue softening with frequency-specific microcurrent. J Al- tern Complement Med. 2013;19:170–177.
5. Curtis D, Fallows S, Morris M, McMakin C. The efficacy of frequency specific microcurrent therapy on delayed onset muscle soreness. J Bodyw Mov Ther. 2010;14(3):272–279.
6. Siskin BF, Walker J. Therapeutic aspects of electromagnetic for soft-tissue healing. In: Blank M, ed. Electromagnetic Fields: Biological Interactions and Mechanisms. Washington, DC: American Chemical Society; 1995:277–285.
7. McMakin C, Chaitow L. Frequency Specific Microcurrent in Pain Management. New York: Elsevier; 2011.
8. Mendell LM. Constructing and deconstructing the gate theory of pain. Pain. 2014;155(2):210–216.
9. Oschman JL. Energy Medicine: The Scientific Basis. New York: Elsevier; 2016.
10. BeckerRO,SeldonG.TheBodyElectric:Electromagnetismand the Foundation of Life. New York: William & Morrow; 1985.
11. Bassett CAL. Bioelectromagnetics in the service of medicine. In: Blank M, ed. Electromagnetic Fields: Biological Inter- actions and Mechanisms. Washington, DC: American Che- mical Society; 1995:265–275.
Address correspondence to:
Stephen Sharp, MD Pain Clinic Department of Anesthesia Walter Reed National Military Medical Center 8901 Wisconsin Avenue Bethesda, MD 20889
E-mail: stephen.j.sharp2.ctr@mail.mil
Downloaded by Walter Reed NMMC (National Military Medical Center) from www.liebertpub.com at 08/07/19. For personal use only.