.
F U L L T E X T S O U R C E : nature
Signals emanating from the nervous system are potent modulators of longevity. It now seems that overall neural excitation is also a key determinant of lifespan.
The question of why and how we age — and why only a minority of humans live to become centenarians — has fascinated people for millennia. Over the past few decades, we have learnt that the rate of ageing is highly sensitive to intrinsic and extrinsic cues, and that these cues act, by means of numerous genetic pathways, to regulate the cellular and systemic processes that ultimately influence ageing1.
Writing in Nature, Zullo and colleagues2 uncover a new twist in the saga: an unexpected link between the nervous system and ageing. They show that overall neuronal excitation is a major determinant of lifespan, and that it is higher in short-lived individuals and lower in the long-lived. The authors also characterize some of the molecular players in this effect, and tie it to a well-known regulator of lifespan: signalling by the hormone insulin or by insulin-like growth factor 1 (IGF1).
Ageing affects the nervous system in a complex way that is not yet fully understood3–5. Perhaps less intuitively, this relationship also works in the opposite direction: signals from the nervous system can modulate the rate of ageing of the whole organism6–10. But although the nervous system is known to influence longevity in species ranging from invertebrates to mammals, the underlying molecular mechanisms have been unclear.
Zullo and colleagues began their investigation by studying brain tissue from aged humans who had shown no cognitive deficits before their death. The authors analysed gene-expression profiles from the frontal cortex, and uncovered an intriguing correlation: genes involved in neural excitation and in the function of the synaptic connections between neurons are downregulated in long-lived individuals, but genes required for inhibitory neurotransmission are not.
How might this occur? The authors found that the downregulated genes are probably targets of the transcriptional regulator protein REST — a general repressor of genes involved in neuronal excitation and synaptic function11. Previous studies12,13 had implicated REST in preventing hyperexcitation of the neuronal network, maintaining its steady state, resisting oxidative stress and protecting neurons over time. (For instance, deleting the Rest gene increases neural activity in the mouse cortex and renders animals vulnerable to blockers of inhibitory neurotransmission, further exacerbating neural excitation and triggering epilepsy.) The new findings directly associate long human lifespan with increased REST activity and reduced neural excitation.
Is this association merely a corollary of the ageing process, or is there a causal relationship? To find out, Zullo and colleagues turned to the nematode worm Caenorhabditis elegans — a malleable test bed that has been invaluable in unpicking the mechanisms that modulate lifespan14. The authors found that neural activity increases as the worm ages. In addition, interventions that inhibit either overall neural excitation and synaptic neurotransmission or signalling by neuropeptide molecules extend the lifespan of C. elegans. In effect, tempering excitatory neurotransmission to reduce overall neural activity is enough to make worms longer-lived. By contrast, suppressing inhibitory neurotransmission increases neural activity and shortens lifespan. Overall neural excitation is, therefore, an important regulator of lifespan in worms and humans.
Digging deeper into this process in worms, the authors focused on the SPR-3 and SPR-4 proteins15, which are counterparts of mammalian REST. This is where the research began to reveal links with insulin/IGF1 signalling, which is a key part of the cellular response to the presence of nutrients. Low insulin/IGF1 signalling is associated with long lifespan in worms.
Zullo et al. found that the longevity conferred by reduced neural activity requires DAF-16, a transcription factor that is also needed for the extended lifespan linked to low insulin/IGF1 signalling in C. elegans. Moreover, neuronal SPR-3 and SPR-4 are key to the increase in lifespan seen under conditions of low insulin/IGF1 signalling. Genes required for neuronal excitation are downregulated by low insulin/IGF1 signalling in an SPR-3/4-dependent manner. In addition, worms carrying mutations in the insulin receptor DAF-2 show reduced neural excitation that is instigated by SPR-3 and SPR-4 and is required for activation of DAF-16. Similarly, SPR-3 and SPR-4 are needed to activate DAF-16 under conditions of oxidative stress. Of note, SPR-3/4 depletion restores higher levels of neural excitation in animals carrying DAF-2 mutations, compromising their exceptional longevity.
Collectively, these findings in C. elegans indicate that stress and insulin/IGF1 signalling converge on SPR-3 and SPR-4 to modulate neural activity. In turn, this influences DAF-16, another point of convergence that integrates neural excitation and insulin/IGF1 signals to promote stress tolerance and longevity (Fig. 1a). Exactly how DAF-16 is activated by reduced neural excitation remains to be seen.
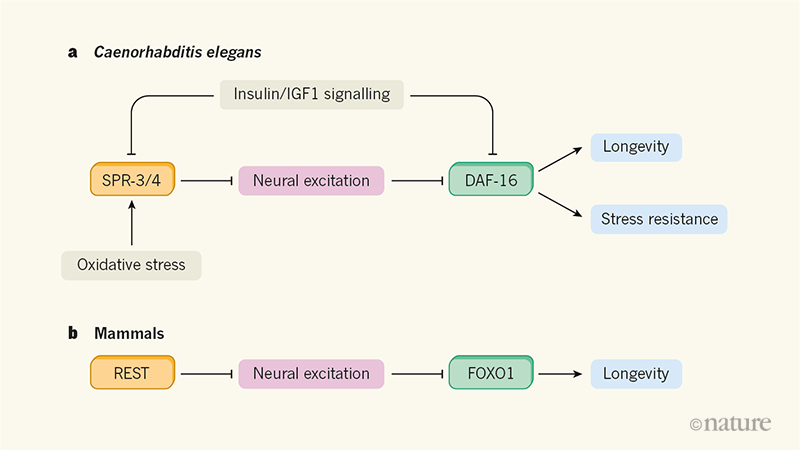
Figure 1 | Lifespan is regulated by neural excitation in worms and mammals. a, Zullo and colleagues2 show that, in the nematode worm Caenorhabditis elegans, the proteins SPR-3 and SPR-4 reduce the expression of genes involved in neural excitation and transmission across synapses. SPR-3 and SPR-4 therefore act to quench neural excitation. Subsequent activation of the transcription factor DAF-16 (normally inhibited by neural excitation) promotes longevity and resistance to oxidative stress. Oxidative stress and signalling through insulin or insulin-like growth factor 1 (IGF1) are both known to affect lifespan. The new findings suggest that they do this, in part, through their effects on SPR-3/4 and neural excitation. b, The authors also find that, in humans and mice, the SPR-3 and SPR-4 counterpart REST downregulates genes involved in neural excitation in the brain’s cortex. The ensuing tempering of neural excitation activates the DAF-16 counterpart FOXO1. REST expression is increased in the cortex of long-lived humans, whereas genes involved in neural excitation are downregulated.
A similar signal-transduction pathway seems to be at work in mammals (Fig. 1b). Zullo et al. find that, in humans, the expression and levels of REST in cell nuclei correlate with those of the DAF-16 counterpart FOXO1. Moreover, both REST and FOXO1 are found in neurons in the human prefrontal cortex. The authors showed that repressing neural excitation in mouse cortical neurons grown in culture increases the expression and nuclear levels of FOXO1. And the age-dependent rise in nuclear FOXO1 in mice requires REST. The parallels between nematodes and mammals suggest that the REST–FOXO1 (or the SPR-3/4–DAF-16) axis is a key part of the mechanisms by which nervous system function influences ageing. Moreover, a reduction in overall neural excitation is a major contributor to the lifespan extension caused by low insulin/IGF1 signalling.
.../...
.










































