
Alternative methods to extend telomeres
#901
Posted 21 May 2023 - 07:38 PM
From there it was just a small jump to seeing telomeres can be well protected by controlling ROS. Many other papers I read over the years that showed me that, some of which I've posted and talked about before. Note here that I'm talking about cells' own ROS production being regulated through mitochondrial mechanisms, not exogenous antioxidants.
My explanation for why telomeres can get longer in downstream cells is obvious, really. It matches all the data. Although white blood cells turn over swiftly and variably, making an absolute length measurement difficult, their responsiveness means we can quickly see the effect of interventions. It is just a pity telomere tests are so expensive and difficult to get.
Perhaps TERC activation can help, I always wanted to combine it with TERT for maximum synergy, though it's not clear we have a reliable way of upregulating TERC. Plus that synergy won't be that effective until we can activate TERT sufficiently.
#902
Posted 04 June 2023 - 05:32 PM
Thanks for the clarification. I will share some brainstorming that I have been doing on this topic, which includes research on two substances that could be effective in reducing ROS-mediated telomere shortening.
1. Follow-up question: Do you believe that, given the interventions currently available to us, the most we can hope to do now with respect to telomere shortening is just greatly slow the rate of shortening? Is true elongation beyond our reach at the moment?
2. Microdosing DNP: You mentioned manipulation of ROS production via mitochondrial mechanisms as a potentially effective way to slow telomere shortening. I would like to bring back to your attention the early stage research on microdosing DNP as a way to induce transient mitochondrial uncoupling with beneficial downstream effects on ROS production, Ca2+ handling, mitophagy, induction of BDNF, and cellular remodeling.
And for compelling anecdotal reports on microdosing DNP, see here
3. Tempol: I think that the antioxidant Tempol (a synthetic SOD mimetic) could help ameliorate ROS mediated telomere shortening. The website HERE is a great compilation for research on Tempol. FYI, Tempol made it to the phase 2/phase 3 stage as a treatment for Covid19. Tempol has shown benefit in studies for a wide range of conditions including infectious diseases, autoimmune diseases, and degenerative diseases of aging. There is also a study showing that Tempol slowed the telomere shortening rate of fibroblasts.
4. Documented results from Epitalon: You have previously mentioned that Epitalon seems promising but lacks quality data to demonstrate its efficacy. Have you seen the drastic results in telomere length that some individuals on reddit have reported from testing their telomere length after administering Epitalon?
#903
Posted 05 June 2023 - 12:23 PM
Thanks for the clarification. I will share some brainstorming that I have been doing on this topic, which includes research on two substances that could be effective in reducing ROS-mediated telomere shortening.
1. Follow-up question: Do you believe that, given the interventions currently available to us, the most we can hope to do now with respect to telomere shortening is just greatly slow the rate of shortening? Is true elongation beyond our reach at the moment?
2. Microdosing DNP: You mentioned manipulation of ROS production via mitochondrial mechanisms as a potentially effective way to slow telomere shortening. I would like to bring back to your attention the early stage research on microdosing DNP as a way to induce transient mitochondrial uncoupling with beneficial downstream effects on ROS production, Ca2+ handling, mitophagy, induction of BDNF, and cellular remodeling.
3. Tempol: I think that the antioxidant Tempol (a synthetic SOD mimetic) could help ameliorate ROS mediated telomere shortening. o source.
4. Documented results from Epitalon: You have previously mentioned that Epitalon seems promising but lacks quality data to demonstrate its efficacy.
1. Yes to both questions. I wish it weren't so.
2 & 3. DNP or Tempol...Maybe. Unsure at this point. We know you give cells Methylene Blue, or NMN or Nicotinamide and something or somethings happen, and the rate of telomere shortening is radically reduced. Insert your reason this is happening <HERE>. Although I have used the explanation of <ROS>, for what I thought at the time was good reasons, I am far from convinced this is an unassailable position. For example, NAD+/NADH ratio is increased, which means the cell is actually in a more oxidised state. So, it may well be that it is downstream genetic expression changes (of the NAD+/NADH ratio) that lead to reduced telomere shortening and other cellular and organism-level benefits. DNP or Tempol may also do this, but also may not. They warrant further investigation.
4. This has already been covered in my previous posts. Epitalon results (which I have seen), NMN results, TAM818 results,...All can be easily explained by the reduction of telomere loss from progenitor to downstream cells, i.e. 10kbp to 5kbp, improved to 10kbp to 7.5kbp - wow, my telomeres have got 50% longer!
Edited by QuestforLife, 05 June 2023 - 12:26 PM.
sponsored ad
#904
Posted 05 June 2023 - 07:24 PM
If we did find a substance that could reliably lengthen telomeres and then applied that substance, what sort of results would we want to find on our telomere tests? Would commercially available telomere tests even be adequate?
Have you come across the research on AGS-499 and its related compounds?
AGS-499 is a small molecule telomerase activator that has been shown in various studies to increase telomerase activity and TERT levels in many human tissues and cells, including neurons and mesenchymal stem cells.
Neuromagen Pharma Ltd., developer of a new class of drugs to treat degenerative and senescence-associated diseases, has received FDA Orphan Drug Designation for AGS-499 its lead drug candidate, which has shown significant effectiveness in delaying the onset and progression of ALS (Amyotrophic Lateral Sclerosis).
Neuromagen's AGS patent-protected family of drugs consists of molecules that activate telomerase reverse transcriptase (TERT). Telomerase is a key enzyme for cell lifespan and survival that prevents telomere shortening and controls cell senescence. The drugs demonstrated preclinical efficacy in ALS and neurodegenerative diseases (Alzheimer's) as well as Diabetes, fertility, and cardiovascular disease.
:text=BEER-SHEVA%2C%20Israel%2C%20May,onset%20and%20progression%20of%20ALS%20(' class='bbc_url' title='External link' rel='nofollow external'>SOURCE
The same group also treated bone marrow-derived human mesenchymal stem cells (hMSCs) with the telomerase activator AGS499 for a prolonged time and found a protective effect against oxidative stress (increased resistance against apoptosis and less DNA damage) as well as a small increase in average telomere length.
https://academic.oup...1/3/233/6795861
Edited by dlewis1453, 05 June 2023 - 07:26 PM.
#905
Posted 06 June 2023 - 11:39 AM
If we did find a substance that could reliably lengthen telomeres and then applied that substance, what sort of results would we want to find on our telomere tests? Would commercially available telomere tests even be adequate?
Have you come across the research on AGS-499 and its related compounds?
AGS-499 is a small molecule telomerase activator that has been shown in various studies to increase telomerase activity and TERT levels in many human tissues and cells, including neurons and mesenchymal stem cells.
NeuromagenCompany Website→ source (external link)
Noveltelomerase-increasing compound in mouse brain delays the onset of amyotrophic lateral sclerosis→ source (external link)
https://www.nature.com/articles/s41598-019-54741-7→ source (external link)
If we did find a telomerase activator that would actually increase telomere length upon cell division, then:
1. this would be evident in cell culture because they would divide indefinitely and TERT levels would be >> HELA (+ve control)
2. it might be detectable in vivo in lymphocytes, with their telomere length reaching a level as great or greater than their progenitors. LifeLength have data on expected telomere length vs age for large populations.
3. Only way to know for sure though would be bone marrow telomere length. Pity Liz Parrish never did this as she had TERT gene therapy which is >>> HELA (in the cells it reaches).
AGS-499, from what I have seen previously (https://doi.org/10.1...598-019-54741-7), is a weak, transitory telomerase activator. That does not mean it will have no benefit, but it will not extend telomeres in a meaningful way.
In terms of directions to look in the future, I'd like to understand what the mechanism is for the reduction of telomere shortening seen with NMN, NAM, MB, etc. It does appear to be related to ROS, but not in a straight forward manner. It probably is related to maintenance of mitochondria. It almost certainly is independent of telomerase, so may be additive with telomerase activators.
#906
Posted 27 June 2023 - 05:17 AM
Some interesting new research has come out that may hint at a new actionable anti-aging strategy. I will try to summarize the research and draw links between the papers in this post.
New research shows that the bowhead whale, which is thought to have a maximum lifespan of over two hundred years, has superior DNA repair capabilities. https://www.biorxiv.....05.07.539748v1 https://www.lifespan...ales-longevity/
analysis of DNA repair revealed that bowhead cells repair double-strand breaks with uniquely high efficiency and accuracy compared to other mammals. Further, we identified two proteins, CIRBP and RPA2, that are present at high levels in bowhead fibroblasts and increase the efficiency and fidelity of DNA repair in human cells. These results suggest that rather than possessing additional tumor suppressor genes as barriers to oncogenesis, the bowhead whale relies on more accurate and efficient DNA repair to preserve genome integrity. This strategy that does not eliminate cells but repairs them, may be critical for the long and cancer-free lifespan of the bowhead whale.
Another new paper showed that injecting mice with the protein Netrin-1, which plays an essential role in maintaining active DNA damage responses, rejuvenates stem cell niches. https://pubmed.ncbi....h.gov/37037837/ https://www.lifespan...-cells-in-mice/
Here, we sought to determine whether aged blood stem cell function can be restored by rejuvenating their supportive niches within the bone marrow (BM). We identify Netrin-1 as a critical regulator of BM niche cell aging. Niche-specific deletion of Netrin-1 induces premature aging phenotypes within the BM microenvironment, while supplementation of aged mice with Netrin-1 rejuvenates aged niche cells and restores competitive fitness of aged blood stem cells to youthful levels. We show that Netrin-1 plays an essential role in maintaining active DNA damage responses (DDR), and that aging-associated decline in niche-derived Netrin-1 results in DNA damage accumulation within the BM microenvironment. We show that Netrin-1 supplementation is sufficient to resolve DNA damage and restore regenerative potential of the aged BM niche and blood stem cells to endure serial chemotherapy regimens.
Interestingly, the Netrin-1 therapy was only applied to mice for about a week, and in quantities that are economically feasible at human scales. It is rare to find a paper in the longevity space that presents such impressive results from a short dosage schedule and at reasonable prices/ quantities. It even seems useful as a treatment to protect DNA from damage during chemotherapy, see below.
The treatment increased the number of functional HSCs following bone marrow transplantation four-fold. Moreover, HSCs from treated aged mice were almost as fit and competent as those derived from young controls.
The researchers also learned that not all beneficial effects of Netrin-1 supplementation on HSCs result from improvements in the bone marrow niche. Many of those effects were recapitulated when HSCs were co-cultured with Netrin-1 in vitro, showing that Netrin-1 probably exerts its benefits both directly and indirectly, via niche improvements.Netrin-1 treatment also significantly protected mice from the detrimental effects of chemotherapy. Treated mice demonstrated preservation of body weight and much more robust hematopoietic recovery. When mice were subjected to an especially damaging multiple-dose chemotherapy, the difference in survival was striking, with the treatment group not losing a single mouse:
I wonder if administering Netrin-1 to a mammal could reproduce some of the longevity of the bowhead whale, by increasing that animal's DNA repair capabilities?
Lastly, we know from David Sinclair's recent paper on the "Information Theory of Aging" that epigenetic degradation is likely downstream of imperfect/inefficient DNA repair. https://www.lifespan...cause-of-aging/ https://www.cell.com...8674(22)01570-7 Sinclair's team prematurely aged mice by hitting them with DNA damage (the repair of which caused epigenetic ages), and then his team was actually able to significantly reverse the mouse's visible age and epigenetic age via epigenetic reprogramming with yamanka factors.
All living things experience an increase in entropy, manifested as a loss of genetic and epigenetic information. In yeast, epigenetic information is lost over time due to the relocalization of chromatin-modifying proteins to DNA breaks, causing cells to lose their identity, a hallmark of yeast aging. Using a system called “ICE” (inducible changes to the epigenome), we find that the act of faithful DNA repair advances aging at physiological, cognitive, and molecular levels, including erosion of the epigenetic landscape, cellular exdifferentiation, senescence, and advancement of the DNA methylation clock, which can be reversed by OSK-mediated rejuvenation. These data are consistent with the information theory of aging, which states that a loss of epigenetic information is a reversible cause of aging.
If a mammal has its DNA repair capabilities improved by Netrin-1, maybe that would have benefits for that mammal's epigenome as well? That mammal might experience significantly slowed epigenetic aging. It would be interesting to see the rate of epigenetic aging of a bowhead whale.
And to tie all this back to telomeres...how could administration of Netrin-1 assist telomere length? My guess is that it would just slow the rate of telomere shortening, but by how much?
#907
Posted 27 June 2023 - 12:03 PM
Some interesting new research has come out that may hint at a new actionable anti-aging strategy. I will try to summarize the research and draw links between the papers in this post.
New research shows that the bowhead whale, which is thought to have a maximum lifespan of over two hundred years, has superior DNA repair capabilities. https://www.biorxiv.....05.07.539748v1 https://www.lifespan...ales-longevity/
Another new paper showed that injecting mice with the protein Netrin-1, which plays an essential role in maintaining active DNA damage responses, rejuvenates stem cell niches. https://pubmed.ncbi....h.gov/37037837/ https://www.lifespan...-cells-in-mice/
Interestingly, the Netrin-1 therapy was only applied to mice for about a week, and in quantities that are economically feasible at human scales. It is rare to find a paper in the longevity space that presents such impressive results from a short dosage schedule and at reasonable prices/ quantities. It even seems useful as a treatment to protect DNA from damage during chemotherapy, see below.
I wonder if administering Netrin-1 to a mammal could reproduce some of the longevity of the bowhead whale, by increasing that animal's DNA repair capabilities?
Lastly, we know from David Sinclair's recent paper on the "Information Theory of Aging" that epigenetic degradation is likely downstream of imperfect/inefficient DNA repair. https://www.lifespan...cause-of-aging/ https://www.cell.com...8674(22)01570-7 Sinclair's team prematurely aged mice by hitting them with DNA damage (the repair of which caused epigenetic ages), and then his team was actually able to significantly reverse the mouse's visible age and epigenetic age via epigenetic reprogramming with yamanka factors.
If a mammal has its DNA repair capabilities improved by Netrin-1, maybe that would have benefits for that mammal's epigenome as well? That mammal might experience significantly slowed epigenetic aging. It would be interesting to see the rate of epigenetic aging of a bowhead whale.
And to tie all this back to telomeres...how could administration of Netrin-1 assist telomere length? My guess is that it would just slow the rate of telomere shortening, but by how much?
The general consensus is that DNA damage is not causal for aging, even though it may happen with aging. Not that the consensus means much at this point. Mechanistically it makes sense it could cause aging, but that doesn't mean it is true. I have suggested before that the damage from DNA repair could be indirect, i.e. from floating pieces of DNA that are taken up by other cells. This certainly seems to happen in a cytokine storm scenario; it might be happening in aging more generally [1]. Others have other ideas. For example, Steve Perry believes that DNA damage is a major cause of stem cell senescence, and that GDF11 reverses this [2]. Sinclair thinks that even successful DNA repair leaves epigenetic marks that lead to aging. Nice idea. But I think this is the weakest idea of the three and am unconvinced by his papers.
You see the problem, though? You have started with a position that DNA damage must be causal to aging and then proceeded from there. It may or may not be the case.
I suspect that many things would kill us eventually if we lived long enough, including inadequate DNA repair. But it is probably not the major reason we age currently. I would say that 90% of aging currently is mitochondrial dysfunction. When that is solved telomeres will probably kill us. After that, maybe DNA damage. But it is anybody's guess at this point because the body is a complex system for which we have no comprehensive theory, hence the value in experimentation, even the most humble experimenter on this site. For this reason I would never discourage you from pursuing Netrin-1 as a treatment. It might just surprise me.
I expect that addressing what mostly kills us now would have an outsized effect, and that is reason for positivity. That is because it should slow many of the other aging processes. For that reason I currently think that mitochondrial treatments will slow telomere loss more than telomerase activators, even though conversely long telomeres can keep mitochondria healthy (though that is not a currently available treatment). The same goes for stem cell attrition and DNA damage.
[1] https://www.medrxiv.....21.20151423v1
[2] https://gdf11rejuvenation.com/
#908
Posted 29 June 2023 - 11:02 PM
1. The general consensus is that DNA damage is not causal for aging, even though it may happen with aging....
2. Sinclair thinks that even successful DNA repair leaves epigenetic marks that lead to aging. Nice idea. But I think this is the weakest idea of the three and am unconvinced by his papers....
3. I would say that 90% of aging currently is mitochondrial dysfunction....
You made three points in your answer that I am curious to discuss further and I am responding to each below.
_________________________________________________________________________________________
1. You mention that you are skeptical of this consensus. Do you think that the Netrin-1 paper, which demonstrated the role that restoring dna damage responses plays in rejuvenating a stem cell niche, is further reason to be skeptical of this consensus? I have included the abstract from the Netrin-1 paper below for reference. These results seem dramatic enough to doubt the consensus, unless it turns out the Netrin-1 is doing other beneficial things besides DNA damage repair, and that these other things are what is rejuvenating the bone marrow stem cell niche.
Aging associated defects within stem cell-supportive niches contribute towards age-related decline in stem cell activity. However, mechanisms underlying age-related niche defects, and whether restoring niche function can improve stem cell fitness, remain unclear. Here, we sought to determine whether aged blood stem cell function can be restored by rejuvenating their supportive niches within the bone marrow (BM). We identify Netrin-1 as a critical regulator of BM niche cell aging. Niche-specific deletion of Netrin-1 induces premature aging phenotypes within the BM microenvironment, while supplementation of aged mice with Netrin-1 rejuvenates aged niche cells and restores competitive fitness of aged blood stem cells to youthful levels. We show that Netrin-1 plays an essential role in maintaining active DNA damage responses (DDR), and that aging-associated decline in niche-derived Netrin-1 results in DNA damage accumulation within the BM microenvironment. We show that Netrin-1 supplementation is sufficient to resolve DNA damage and restore regenerative potential of the aged BM niche and blood stem cells to endure serial chemotherapy regimens.
_________________________________________________________________________________________
2. Whether or not DNA damage itself is causal for aging, it may still be the case that repair of DNA damage is causal for aging, as asserted by Sinclair. I am very interested to hear your thoughts on why you are unconvinced by Sinclair's theory that the faithful repair of dna damage leads to detrimental epigenetic alterations that drive aging ("Information Theory of Aging"). Personally, I thought his recent paper was very compelling, but I am not a scientist. I looked up some cautionary statements by other scientists commenting on Sinclair's recent paper, and have pasted them below.
To see whether the epigenetic degradation was reversible, the researchers injected some of these elderly seeming mice with AAVs carrying OSK genes, which Sinclair’s group recently reported could reverse loss of vision in aging rodents. Analyses of the mice’s muscles, kidneys, and retinas suggest the cocktail reversed some of the epigenetic changes induced by the DNA breaks. The findings point to a way to drive an animal’s age “forwards and backwards at will,” Sinclair says, and support the idea of epigenome-targeting treatments for aging in humans.
Molecular biologist Wolf Reik, director of the Altos Cambridge Institute of Science (opened last year by rejuvenation-focused company Altos Labs), praised the sophistication and thoroughness of the Harvard team’s study, but says the team’s indirect way of inducing epigenetic changes with dramatic DNA breaks that could have other effects makes it hard to prove those changes are what’s causing aging. It’s also unclear how well mice with induced DNA breaks mimic naturally aging animals," says Jan Vijg, a geneticist at the Albert Einstein College of Medicine.He and others stress that aging is a complex process with multiple contributing factors, and that in both papers, the effects of OSK treatment were moderate: a small extension of life span in one, and a partial reversal of artificially induced symptoms in the other. “The jump that now aging is a program” that can be wound backward isn’t justified by the research, Vijg says.
https://www.science....igns-aging-mice
_________________________________________________________________________________________
3. What type of mitochondrial dysfunction are you referring to? Is it primarily accumulation of mtDNA mutations? If so, would the latest version of Turnbuckle's fission-fusion mitochondrial protocol help us in this area, assuming it works as he described? Anecdotally, multiple people in that thread claimed to experience significant benefit.
_________________________________________________________________________________________
Lastly, I know that you are currently researching the importance of NAD+ in preserving telomere length. It seems we could attack this from several angles: (i) reduce mtDNA driven mitochondrial dysfunction with Turnbuckle's fission-fusion protocol, which should increase endogenous NAD+ production, (ii) introduce exogenous NAD+ via supplementation, (iii) increase NAD+ recycling by increasing expression of NAMPT, and (iv) reducing CD38 by reducing senescent cell burden and decreasing chronic inflammation. This supplement company (https://nuchido.com/...-generation-nad) has a product that attempts to boost NAD+ through angles (ii),(iii),and (iv) and they list their ingredients and the reasoning behind them. It could be useful as a roadmap.
#909
Posted 30 June 2023 - 08:15 AM
You made three points in your answer that I am curious to discuss further and I am responding to each below.
_________________________________________________________________________________________
1. You mention that you are skeptical of this consensus. Do you think that the Netrin-1 paper, which demonstrated the role that restoring dna damage responses plays in rejuvenating a stem cell niche, is further reason to be skeptical of this consensus? I have included the abstract from the Netrin-1 paper below for reference. These results seem dramatic enough to doubt the consensus, unless it turns out the Netrin-1 is doing other beneficial things besides DNA damage repair, and that these other things are what is rejuvenating the bone marrow stem cell niche.
_________________________________________________________________________________________
2. Whether or not DNA damage itself is causal for aging, it may still be the case that repair of DNA damage is causal for aging, as asserted by Sinclair. I am very interested to hear your thoughts on why you are unconvinced by Sinclair's theory that the faithful repair of dna damage leads to detrimental epigenetic alterations that drive aging ("Information Theory of Aging"). Personally, I thought his recent paper was very compelling, but I am not a scientist. I looked up some cautionary statements by other scientists commenting on Sinclair's recent paper, and have pasted them below.
_________________________________________________________________________________________
3. What type of mitochondrial dysfunction are you referring to? Is it primarily accumulation of mtDNA mutations? If so, would the latest version of Turnbuckle's fission-fusion mitochondrial protocol help us in this area, assuming it works as he described? Anecdotally, multiple people in that thread claimed to experience significant benefit.
_________________________________________________________________________________________
Lastly, I know that you are currently researching the importance of NAD+ in preserving telomere length. It seems we could attack this from several angles: (i) reduce mtDNA driven mitochondrial dysfunction with Turnbuckle's fission-fusion protocol, which should increase endogenous NAD+ production, (ii) introduce exogenous NAD+ via supplementation, (iii) increase NAD+ recycling by increasing expression of NAMPT, and (iv) reducing CD38 by reducing senescent cell burden and decreasing chronic inflammation. This supplement company (https://nuchido.com/...-generation-nad) has a product that attempts to boost NAD+ through angles (ii),(iii),and (iv) and they list their ingredients and the reasoning behind them. It could be useful as a roadmap.
1. I am always suspicious of the consensus in any field, as it too easily becomes a self-reinforcing belief that bars further progress. I wasn't being specific to DNA damage or Nestrin. Basically experiment is king, so show me sufficient evidence and I'll change my mind (but not about being suspicious of an easy consensus).
2. I believe Sinclair was just killing lots of cells through his DNA damage experiments. So nothing solid can be drawn from the experiments regarding DNA damage being causal for epigenetic change. But sure, OSK(M) may be very beneficial in aging, possibly.
It is too easy with animal models to make a proxy for a human disease, like dementia in mice say, and then cure that, and think you've cured dementia. You haven't, you've just resolved something with similar symptoms. Smash up the DNA of an animal, or kill lots of its cells, and of course you are going to get something that passably looks like aging. But is it?
Also, the anti-aging field is now attracting attention from people out to make a lot of money. I don't want to be too harsh to Sinclair, but my spidey sense has being going off on him for some time. Not that I think scientists and entrepreneurs shouldn't be able to make money in this space, they should, but if that becomes the primary goal, we have a problem. I think it will be a while before the dust settles on Sirtuin science, and we see what was real and what was not.
3. My opinions on mitochondrial aging are purely pragmatic. It was clear to me that interventions here were more effective at protecting telomeres than (weak) telomerase activators themselves, which also tend to me much more expensive. Also, I've been reading papers on aging for over 10-years and it was clear to me that outsized effects were cropping up now and again with interventions pertaining to the mitochondria. These weren't always easily replicable, i.e. glycine, C60, cysteine, glutathione, catalase, etc, but they were interesting. The problem with mitochondrial aging is that the pathways are fiendishly complicated. I've got an idea that despite my reservations on Sinclair, he actually is on the right track with the NAD+/NADH ratio, only boosting NAD+ doesn't seem like an optimal strategy to me.
#910
Posted 04 July 2023 - 08:05 PM
How is your longevity protocol looking now QuestforLife? Are you still taking AKG and GDF11 and rock inhibitor eye drops? Any change aside from dropping the telomerase boosting supplements?
#911
Posted 06 July 2023 - 10:01 AM
How is your longevity protocol looking now QuestforLife? Are you still taking AKG and GDF11 and rock inhibitor eye drops? Any change aside from dropping the telomerase boosting supplements?
Haven't taken GDF11 in months. May use it later in the year.
I still take AAKG either in liposomal or powder in water (most days).
I stopped the rock inhibitor drops as they were causing my throat to become slightly swollen and making me snore. I may use them again at some point, in moderation.
Brief Update: I am experimenting with other mitochondrial supplements. But this has been complicated by hayfever season, which is made much worse by anything that boosts NAD+ too much.
My general feeling is that increasing NAD+ is not the way to go, as NAD+:NADH is obviously massively biased to NAD+ anyway (except in very sick cells), which explains why you have to take a lot of NAD+ to experience big benefits (this is especially obvious in mice studies). Oxidising NADH seems like a better plan and way to go. But this has lead me back down the metabolic rabbit hole I explored several years ago with my high saturated fat diet. That diet eventually killed my energy, but I probably was on the right track, as this is what you'd expect from lower NADH and higher metabolic rate. Again, it seems that all roads lead to calorie restriction.
#912
Posted 16 July 2023 - 11:55 AM
dlewis1453: "Interestingly, the Netrin-1 therapy was only applied to mice for about a week, and in quantities that are economically feasible at human scales. It is rare to find a paper in the longevity space that presents such impressive results from a short dosage schedule"
reasonable? 630€ for 10ug....a rip off to me
Edited by Young Paul, 16 July 2023 - 11:58 AM.
#913
Posted 16 July 2023 - 08:59 PM
reasonable? 630€ for 10ug....a rip off to me
That price you quoted is one of the higher prices. 25 ug of Netrin-1 is available for $394 from other vendors and that does not take into account bulk discounts, which can be significant. For example, I wouldn't be surprised if 100 ug of Netrin-1 could be acquired for around $800,and 1mg of Netrin-1 for around $5,000. Whether or not this is a reasonable price is dependent on the context. Once you have looked around enough at the prices of experimental proteins that are sold for research purposes, you will see that this pricing is not bad compared to most and is feasible at the doses required.
In the study I quoted, Netrin-1 was administered to mice in 10 doses of 4 ug and it rejuvenated their blood stem cell niche and made their blood stem cells behave like those of young mice. That's a dramatic result that no currently publicly available substance is able to match. An equivalent human protocol of Netrin-1 could be about 100 ug x 10 doses, which would be about $5,000 with my hypothetical pricing. Maybe to be safe and also save money we halve it to 50 ug x 10 doses for about $3,000. I think many people would be willing to spend $3,000 to rejuvenate their blood stem cells over a period of 2 weeks, if they new it was safe and would work.
This is a high risk experiment though, since Netrin-1 has never been tested in humans. Spend $3,000 and potentially restore your blood stem cells (and potentially other stem cells too) to a youthful level of activity, or potentially get not benefit (or suffer side effects) and be out $3,000.
#914
Posted 16 July 2023 - 09:15 PM
One step closer to creating large amounts of lab-grown meat economically - using immortalized stem cells!
https://scitechdaily...xpand_article=1
Researchers at the Tufts University Center for Cellular Agriculture (TUCCA) have made strides toward this objective by developing immortalized bovine muscle stem cells (iBSCs). These cells possess a rapid growth rate and the ability to divide hundreds of times, potentially even indefinitely, furthering the potential for large-scale meat production.
Normal muscle stem cells drawn from live animals to start a culture typically divide only about 50 times before they start to get “old” and are no longer viable. While it is theoretically possible for these stem cells to produce a substantial amount of meat, the immortalized cells developed by the TUCCA team offer several advantages. One is the possibility of producing significantly more mass for meat production.
Two steps were key to transforming regular bovine muscle stem cells into the immortalized bovine muscle stem cells. Most cells, as they divide and age, begin to lose DNA at the ends of their chromosomes, which are called telomeres, like worn ropes that get frayed with use. This can lead to errors when the DNA is being copied or repaired. It can also cause genes to be lost and, eventually, cells to die. The researchers engineered the bovine stem cells to constantly rebuild their telomeres, effectively keeping their chromosomes “youthful” and ready for another round of replication and cell division.The second step to immortalizing the cells was to make them continuously produce a protein that stimulates a critical stage of cell division. This effectively turbocharges the process and helps the cells to grow faster.
The most interesting part for our research is the fact that these immortalized stem cells have been engineered to maintain their telomere length. I thought this was a good reminder that we should be alert for ways to selectively lengthen the telomeres of stem cells, as has been discussed earlier in this thread.
I was also wondering if someone's own stem cells could be harvested, engineered to express telomerase in the manner of these meat producing immortalized stem cells, and then reinserted into the person.
#915
Posted 17 July 2023 - 01:13 AM
#916
Posted 18 July 2023 - 10:22 AM
One step closer to creating large amounts of lab-grown meat economically - using immortalized stem cells!
https://scitechdaily...xpand_article=1
The most interesting part for our research is the fact that these immortalized stem cells have been engineered to maintain their telomere length. I thought this was a good reminder that we should be alert for ways to selectively lengthen the telomeres of stem cells, as has been discussed earlier in this thread.
I was also wondering if someone's own stem cells could be harvested, engineered to express telomerase in the manner of these meat producing immortalized stem cells, and then reinserted into the person.
It has been possible to immortalise cells for decades. The only thing new here is that those immortalised cells are muscle cells. So you can grow a fake steak without worrying about the cells giving up after a few tens of divisions. I discussed at length before using conditional reprogramming factors to do a similar thing - but much more safely - as soon as the factors are removed the cells differentiate and can senesce, if appropriate. Much as I like the idea of my stem cells being immortal, I wouldn't trust them to do it property - they usually insert viral genes to accomplish the TERT expression, and there doesn't seem to be much control in where it goes.
#917
Posted 31 August 2023 - 09:40 PM
A fascinating new paper (https://www.nature.c...598-023-39370-5) was recently released which found that extracellular vesicles are largely responsible for the rejuvenating effects of young plasma. I have pasted the abstract of the paper below for reference.
Of particular interest to this thread is the fact that extracellular vesicles were able to lengthen the telomeres of cardiac cells "After 4 monthly systemic injections, the hearts of CDC-EV-treated rats, but not control rats, showed signs of rejuvenation: telomere length (~two-fold increase, p < 0.001; Fig. 1B,C)."
Abstract:
Rejuvenation of an old organism was achieved in heterochronic parabiosis experiments, implicating different soluble factors in this effect. Extracellular vesicles (EVs) are the secretory effectors of many cells, including cardiosphere-derived cells (CDCs) with demonstrated anti-senescent effect. 1. To determine the role of EVs (versus other blood fractions) on the rejuvenating effect of the young blood. 2. To evaluate the anti-aging properties of therapeutically administered EVs secreted by young-CDCs in an old organism. Neonatal blood fractioned in 4 components (whole blood, serum, EV-depleted serum and purified EVs) was used to treat old human cardiac stromal cells (CSPCs). CDCs were generated from neonatal rat hearts and the secreted CDC-EVs were purified. CDC-EVs were then tested in naturally-aged rats, using monthly injections over 4-months period. For validation in human samples, pediatric CDC-EVs were tested in aged human CSPCs and progeric fibroblasts. While the purified EVs reproduced the rejuvenating effects of the whole blood, CSPCs treated with EV-depleted serum exhibited the highest degree of senescence. Treatment with young CDC-EVs induce structural and functional improvements in the heart, lungs, skeletal muscle, and kidneys of old rats, while favorably modulating glucose metabolism and anti-senescence pathways. Lifespan was prolonged. EVs secreted by young CDCs exert broad-ranging anti-aging effects in aged rodents and in cellular models of human senescence. Our work not only identifies CDC-EVs as possible therapeutic candidates for a wide range of age-related pathologies, but also raises the question of whether EVs function as endogenous modulators of senescence.
Around the same time, Harold Katcher, his business partner Sanjay, and Steve Horvath published a paper (https://www.biorxiv....552148.full.pdf) detailing how their plasma fraction "E5" was able to greatly reverse epigenetic age in various organs. Interestingly, this paper confirmed that the active component of E5 is the exosomes (a sub-type of extracellular vesicles). This paper did not mention telomeres, but it provides further confirmation of the rejuvenative potential of extracellular vesicles.
The plasma fraction treatment described here is a step change from HPE, as it uses neither whole blood nor plasma, but the exosome fraction of the plasma, which we term as E5
Based on these two papers, it appears that extracellular vesicles are able to rewind both the telomere and epigenetic clocks!
Human umbilical cord plasma is one potent source of extracellular vesicles that is currently available, although it is a bit pricey. This paper (https://pubmed.ncbi....h.gov/36052758/) found that 10 injections of 1 ml of cord plasma concentrate (equivalent to 100 ml of unconcentrated cord plasma) was able to reverse epigenetic age of elderly humans by .82 years on average, with no significant increase in telomere length. More than 20 other clinical biomarkers were significantly and beneficially altered following the treatments. This protocol's modest effect on epigenetic age and telomere length could be due to the small size of the total dosage amount and perhaps the fact that the plasma was injected intramuscularly rather than intravenously.
Going forward, I expect that extracellular vesicles harvested from young plasma will drop in cost and become more accessible. Since the first two papers I cited suggest that plasma from young mammals has a safe, rejuvenating effect in other species of mammals, it should be possible to harvest plasma from young pigs, young cows, etc.
Edited by dlewis1453, 31 August 2023 - 09:42 PM.
#918
Posted 01 September 2023 - 01:00 PM
In terms of the telomere findings, there are several things worth noting. Firstly the rats had short telomeres in their hearts! As these are typically not dividing cells, this means they are losing telomeres through direct damage. Secondly, the benefits to telomere length came from rat exosomes. They have no idea what was in those exosomes. We know these kind of vesicles can contain telomeres (I posted a while back on this happening in the human immune system, where b cells donate to t cells). We also know (mouse embryonic) cells provide telomerase to human cells undergoing conditional reprogramming (I'll add all the links when I'm not on my phone). Pigs do have active telomerase, so we might expect a telomerase benefit from E5. Even human plasma might be of benefit, if it contains exosomes containing telomere fragments, which we know can be taken up and used by other cells.
But finally a note of caution. We know BAD things are also transmitted this way,for example cell free chromatin. This is probably the main reason old blood is so bad for you. I'd be a little worried about what other things might be in porcine exosomes.
#919
Posted 02 September 2023 - 05:44 AM
I am not a patent lawyer, but I think their patent may still be accepted since the use of porcine plasma for this purpose is novel. Maybe human umbilical cord plasma would be safer for now. This is currently available but a bit pricey at the dosages that are likely required for significant effects.
As these are typically not dividing cells, this means they are losing telomeres through direct damage.
Could you elaborate a bit on this point?
I am very excited about this discovery. What about you? It is very promising to find something that appears to repair epigenetics, lengthen telomeres, and improve health biomarkers all at once.
#920
Posted 02 September 2023 - 10:53 AM
In terms of the telomere findings, there are several things worth noting. Firstly the rats had short telomeres in their hearts! As these are typically not dividing cells, this means they are losing telomeres through direct damage. Secondly, the benefits to telomere length came from rat exosomes. They have no idea what was in those exosomes. We know these kind of vesicles can contain telomeres (I posted a while back on this happening in the human immune system, where b cells donate to t cells). We also know (mouse embryonic) cells provide telomerase to human cells undergoing conditional reprogramming (I'll add all the links when I'm not on my phone). Pigs do have active telomerase, so we might expect a telomerase benefit from E5. Even human plasma might be of benefit, if it contains exosomes containing telomere fragments, which we know can be taken up and used by other cells.
But finally a note of caution. We know BAD things are also transmitted this way,for example cell free chromatin. This is probably the main reason old blood is so bad for you. I'd be a little worried about what other things might be in porcine exosomes.
Still on my phone, but...
Reference to post of donation of telomeres in human immune system is here: https://www.longecit...-11#entry899161
Comments on senescent feeder cells supplying telomerase is here:
https://www.longecit...es/#entry864534
Comments on being poisoned by cell free chromatin is here:
https://www.longecit...-30#entry920902
I'll reply in detail about the telomere shortening in heart cells later.
#921
Posted 02 September 2023 - 05:49 PM
Oxidative Stress in Rat Hearts
Regarding the shortened telomeres (subsequently lengthened) in the hearts of rats in the exosome paper [1].
Figure B and the histogram C below it, shows a large increase in telomere length when exosomes are injected. As the exosomes are injected into a chamber of the heart, we'd expect the greatest effect here (although it would have been nice to see the effect on telomere length in other tissues).
But more to the point, given heart cells don't (often) divide, why are they shortened in the first place? What is going on?
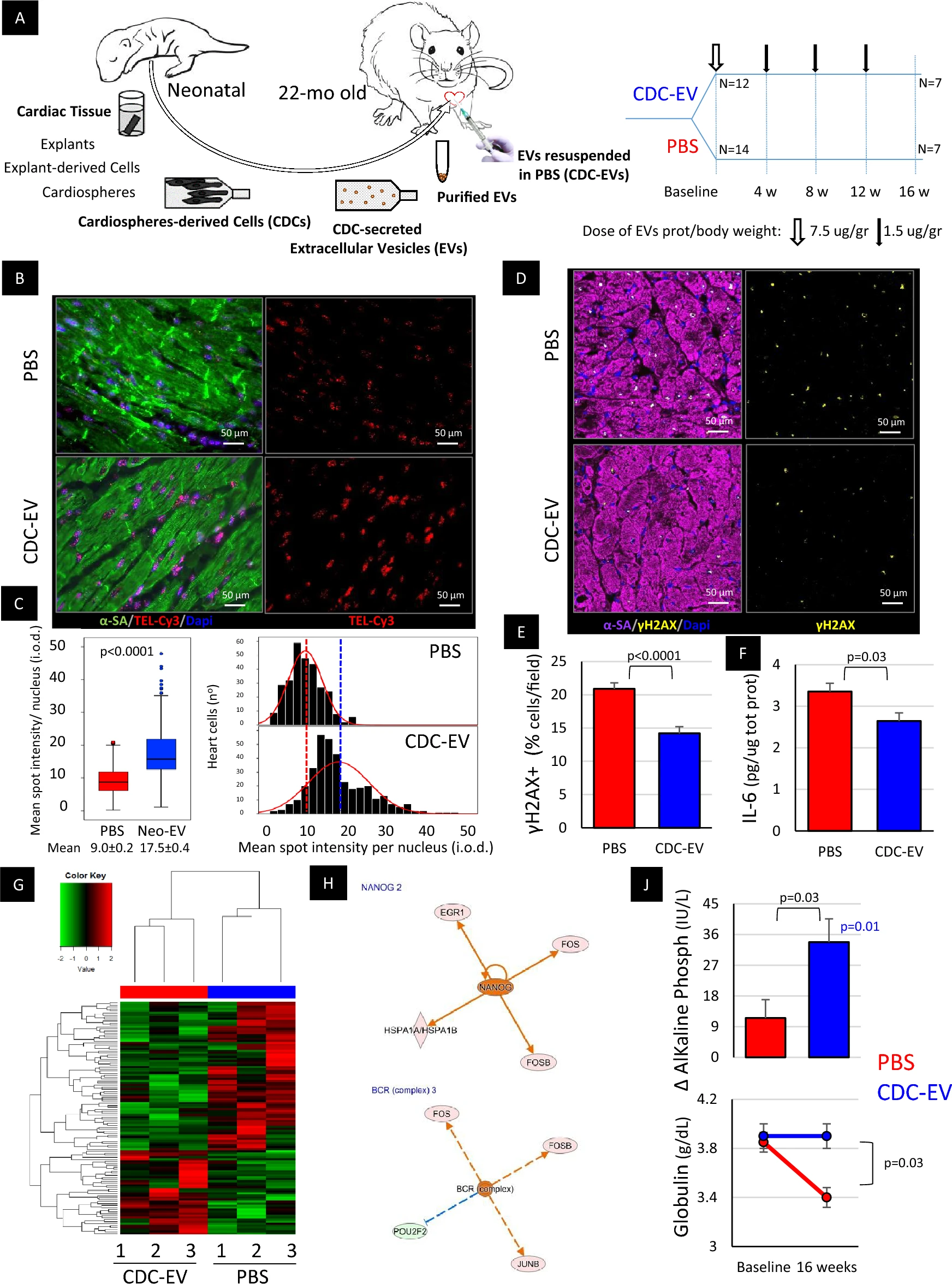
We've discussed on this thread before about the contribution to telomere shortening made by oxidative stress as opposed to cell division. It is likely that what is happening here and that this is proof this happens in vivo, at least in oxidative stress prone rats. .
A DNA damage response (DDR) is triggered when double strand breaks expose DNA without the telomere cap. If this happens, then as quickly as possible this must be recognised and the two ends joined back together. If this happens within the TTAGGG repeats of the telomere, presumably this doesn't happen? This makes sense, as telomeres have a whole bunch of 'shelterin' proteins, whose main function is to prevent the telomere end being recognised as broken DNA. In that case you'd expect that a double strand break in the telomere would result in the telomere being curled back up without adding the broken strand back on, like a child holding a injured arm to their body and not letting you look at it.
I couldn't find much on this with a quick Google search, but what little I found seem to support this idea; for example [2].
This does then beg the question, how do those T cells receive telomere 'donations' from those helpful antigen presenting cells (as discussed previously [3]), if the telomere is protected from end to end joining? My best guess is that during S-phase, when the cell is dividing (like T cells do ALOT), then the telomere can be added to (as it is by telomerase) and this isn't recognised as a double strand DNA break. Of course the details of exactly how this works are yet to be worked out.
By I digress... the important thing here is that rat hearts seem to be under severe oxidative stress.
[1] Grigorian Shamagian, L., Rogers, R.G., Luther, K. et al. Rejuvenating effects of young extracellular vesicles in aged rats and in cellular models of human senescence. Sci Rep 13, 12240 (2023). https://doi.org/10.1...598-023-39370-5
[2] AUTHOR=Ackerson Stephanie M., Romney Carlan, Schuck P. Logan, Stewart Jason A. , To Join or Not to Join: Decision Points Along the Pathway to Double-Strand Break Repair vs. Chromosome End Protection, Frontiers in Cell and Developmental Biology, 2021, DOI=10.3389/fcell.2021.708763
Edited by QuestforLife, 02 September 2023 - 05:56 PM.
#922
Posted 10 October 2023 - 01:01 PM
Summary of ‘Alternative Methods to Extend Telomeres’ Sept 2018 to Oct 2023
Early work on NAD+
https://www.longecity.org/forum/topic/102169-alternative-methods-to-extend-telomeres/#entry857309
SIRT4
Loss of NAD+ because of telomere shortening
Work on Statin-Sartan protocol
https://www.longecity.org/forum/topic/102169-alternative-methods-to-extend-telomeres/#entry862269
link between ROCK inhibitors and telomerase
https://www.longecity.org/forum/topic/102169-alternative-methods-to-extend-telomeres/#entry864097
possible link with senolytics
https://www.longecity.org/forum/topic/102169-alternative-methods-to-extend-telomeres/#entry864534
using ROCK and mTOR inhibitors to reprogram brain cancer cells into normal neurons
https://www.longecity.org/forum/topic/102169-alternative-methods-to-extend-telomeres/#entry865160
How ROCK inhibitors block differentiation
Feedback on protocol
Summary of ROCK inhibition action on cells
Attempts to come up with alternatives to statin and sartans
Diagram of interventions
Paper linking up ROCK and ECM
ROCK and tgf-b
Mean and Max lifespan extension with a ROCK inhibitor
Work on telomerase activators and other important telomere papers
Royal Jelly
Review of various activators
Asiaticoside
Some other telomeres studies
Effect of antioxidant on telomere shortening in the bone marrow
More on the same, later
Telomere activators and CV diseases
Telomere shortening predicts species life span
using TERC upregulation to increase telomere length in stem cell
Telomerase and Splicing Factor regulators
T cells taking telomere length from other cells
Do stem cell stimulants deplete the bone marrow pool?
Hyperbaric oxygen therapy
Discussion of Blasco paper on hyperlong telomere mice
Discussion of actual in vivo rate of telomere attrition
GDF11 lengthen telomeres in MSCs via TERC upregulation
Possible benefit of Klotho to telomeres
Nucleotides (specifically guanine) for elongation of telomeres: eat Anchovies and Herring!
Blasco and short telomeres in kidney disease plus possible connection of short telomeres and the cancer causing epithelial to mesenchymal transition
What is the most powerful telomerase activator and a comparison of methods of measurement
Melatonin is the best antioxidant for telomeres?
More on melatonin
AKG and telomere length (in mice)
Discussion of a cell permeable, oxidation resistant form of Vit C and telomeres plus follow on discussion of ROS hormesis in some cell types
Various discussions on the bioavailability of Asiatic acid/asiaticoside (a purported telomerase activator) and why you may only want a very small dose
Should we be taking Zinc for our telomeres?
Clear benefits to life expectancy, CVD and Cancer with longer telomeres: a study with 500k people
Ability of endothelial cells to make new lining is telomere length dependent
Caffeine promotes telomerase expression
Dark chocolate for telomeres
Alternatives to a telomere test: NLR and CRP
Hyperfunctional telomerase: do you want more cell division or longer telomeres?
We should be aiming for mouse levels of telomerase, not HELA levels
New intranasal and injectable gene therapy for healthy life extension
New GDF11 telomerase paper in Nature:
Growth differentiation factor 11 attenuates cardiac ischemia reperfusion injury via enhancing mitochondrial biogenesis and telomerase activity
Telomerase increases mitophagy through PINK1 - explanations for my increased exercise tolerance
Polymorphic tandem DNA repeats activate the human telomerase reverse transcriptase gene
Telomere length and telomerase activity in T cells are biomarkers of high performing centenarians
Caffeine promotes the expression of telomerase reverse transcriptase to regulate cellular senescence and aging
Are the oncogenic effects of telomerase mediated by methyl transferases?
Loss of GDF11 shortens telomeres
Does Berberine shorten telomeres?
Mitochondrial Telomerase Reverse Transcriptase Protects from Myocardial Ischemia/reperfusion Injury
Naringenin: super supplement?
NMN increases telomere length
Has the telomere theory of aging been proven?
Telomerase is made in the nucleolus
Telomerase protein levels as measured by immunohistochemistry are unreliable
Telomere Shortening in Hematopoietic Stem Cell Transplantation: A Potential Mechanism for Late Graft Failure?
The flaws of methylation clocks
What is Tianshengyuan-1?
Supertelomerase!
Final telomerase protocol
Adding telomerase to symmetric division: a simplified model
Spermidine
Can ultrasound overcome replicative senescence?
Should we take SAMe for telomeres?
Can NO (nitric oxide) increase telomerase activity?
DHA
What is the optimal strategy for slowing telomere loss? It is not telomerase activators
Oxidative Stress in Rat Hearts: a tale of exosomes
View of Aging
Importance of cell size
The Selfish Cell lives longer
Telomeres are NOT passive in aging
Discussion of telomeres and cancer
Senescence and Cancer, again
Are methylation changes with age evidence of a program?
Comments on heterochronic parabiosis
More on Selfish Cell theory of aging (2021)
Age related methylation and the connection with the Selfish Cell Theory of Aging
Plus why aging is cancer
Putting together telomere and hyperfunction theories of aging
Oxidative stress alters global histone modification and DNA methylation
How non-differentiating Selfish (stem) cells come to dominate the stem cell pool; links between methylation, telomerase and ROS
Finding the Culprit: the hormones required for sexual maturity may be the trigger that starts aging via downregulation of TET2
Discussion over whether methylation of gene promoters is protective against stem cell loss and the counter evidence: immortalised cells accumulate such methylation
Discussion of the combined use of telomerase activators, GDF11, AKG, vit A and C
Summing up the Twin Evils of aging
The Evolution of the Selfish Cell
Cancer and the Selfish Cell
Does the Selfish Cell imply programmed or accidental aging?
Finding the Culprit II: Species' cellular ROS level sets aging rate via down regulation of demethylases and failure of Circadian Rhythm
How does the Selfish Cell affect post-mitotic cells?
Why long telomeres won’t make you live forever, but short telomeres mean you’ll die young
Defining the steps that lead to cancer
What are epigenetic Aging tests actually measuring?
A new thesis
The Secret to lengthening telomeres in all cells
The Tortoise and the Hare
Hyperfunctional Telomeres Part II
Paving the way for the 'speeding car': Explaining the benefits of long telomeres in the context of mTOR
Just how much can we inhibit mTOR?
A reanalysis of some epitalon studies and a new take on the DNA mutation theory of aging
Are we being poisoned?
Skin aging
Stem cell competition – can you have too much symmetrical division?
Results
Methylation results from Statin-Sartan protocol
Telomere length improvements via Lifelength
PhenoAge improvements
Epitalon increases methylation age and discussion
Further discussion
Plan to reduce both telomere and methylation age
No improvement in methylation age from 3 months of AKG
Improvement in methylation age from 6 months of AKG
Further improvement in epigenetic age (-6.6 years)
Summary of GDF11 experience with biomarkers
May 2021 Methylation age results
Discussion of reaction times on GDF11
October 2021 Trume Results
Amino Acid results: Do telomerase activators deplete glutamine?
February 2022 Trume Results
Is it time to abandon methylation based aging tests?
Results: March 2023
a theory for why epigenetic aging is transitory:
Sundry
Fatty Acid Oxidation
Starvation and stem cell renewal
See other thread:
Feeding stem cells: the strange case of dietary restriction and alpha lipoic acid
Possible use of pioglitazone with telomerase activators to increase subcutaneous fat without bladder cancer risk
Resveratrol is weird.
Demethylating the klotho promoter with hydrogen sulphide
Melatonin is linked to mitochondrial function and increases TET2 production
Discussion starting here on reversing thymic involution
Comment on gonadal rejuvenation by GDF11
Mitochondrial hyperfusion via metabolic sensing of regulatory amino acids
Edited by QuestforLife, 10 October 2023 - 01:04 PM.
#923
Posted 12 October 2023 - 02:25 PM
My current thoughts on aging, October 2023
A simplified explanation of aging:
-
Lots of cells die daily;
-
Cells will eventually run out of replacements, so long before this they reduce the replacement rate;
-
Various other (mal)adaptations occur in the body.
I will try and elaborate on 1-3, in each of the following paragraphs.
The main difference between cell aging in a petri dish and in a mammal, is that in a petri dish the culture is divided every time the dish becomes full of cells, so the influence of dying cells is diluted. Even so, their effect is felt by the remainder, but it is muted compared to what is experienced in a real body. A body is having to deal with the constant daily death of hundreds of billions of cells. Their toxic products, primarily cell free chromatin, is a significant source of (premature) aging in the rest of the body. Fortunately stopping, or at least reducing, this damage seems quite easy, now we know it’s a problem. There are other benefits to this strategy, related to infection and inflammation, which I won't go into here.
If we are able to prevent, or at least reduce the effects of dying cells, we will slow death in the remainder, which will somewhat delay the hard limit that short human telomeres place on cellular division and replacement. But eventually this process will cause aging and death regardless. Therefore we must do all we can to reduce telomere loss, and ideally, return them to a youthful length. No telomerase activators are currently able to do this, outside of gene therapy. Nevertheless telomerase activators may still be beneficial in reducing telomere loss and also enabling other rejuvenation therapies to be successful. There are also other interventions that do not target telomerase that nevertheless reduce telomere shortening, quite probably more effectively than currently available small molecule telomerase activators.
We all know the downstream consequences of aging, and they suck. Various celebrities or other rich folk attempt to reverse these effects, with growth hormones, stem cells, and other related treatments, mainly focussed in restoring youthful signaling and growth like factors. Surprisingly, these treatments do seem to have some benefit, albeit far from sufficient to restore youth. It should be expected that if we address 1 and 2, then treatments category 3 should become much more effective.
#924
Posted 17 October 2023 - 02:22 PM
Yuri Deigin's article on epigenetic aging and methylation clocks, with interesting references
This article is really interesting.
But why is advantageous to kill off older individuals ?!? From egoist gene point of view no sense... just a waste of reproductive potential. We miss something. IMHO the point is we are a MIX of human DNA + mithocondrial DNA + intestine bacterial DNA.... and ALL this DNA want to replicate collaborating. What tears off ? By my opinion is mithocondria. The rest of DNA (the humane one) just is programmed to keep in pace with mitocondrial degenerations.... so changes in both DNA methilations and telometer lengt (and maybe other clocks) are just needed to cope with LESS ATP and much more dirty functioning of mitocondria. This and probably transposons awakening and probably also bad pieces of waste DNA around that spoils the DNA expression. Methilations is an adaptation to other bad factors. Like having a car with brakes broken: you forced to drive slowly... and drive slowly can let you go on.... but problem is not that you go slow, problem is in the broken brakes.
Edited by HBRU, 17 October 2023 - 02:26 PM.
#925
Posted 17 October 2023 - 02:47 PM
Here's a much simpler explanation. It is the stochastic damage of metabolism that makes epigenetic clocks tick. Old cells (without ready replacements) acquire dysfunctional mitochondria through stochastic metabolic processes and the passage of time The cell then adapts to a bad situation by making the best of it and down and upregulating various genes in the nucleus. Then (naive) scientists come along and decide the problem (and aging itself) is those adaptations.
Consider this the null hypothesis. Disprove it if you can.
same opinion.... well you can even feel is true... you feel that while in mito-fission you simply "feel" older.... :D... and if you keep many days in mito-fission state then you got even permanent health trouble as DNA adapt in metilation to a less efficient energy production state (it keeps brakes so you dont damage yourself by excessive bad mitocondira work).... so good to take AKG while in mito fission (to avoid bad metilations).... and good not make too much mito-fissions.... just what is necessary for cleaning out bad ones....
Edited by HBRU, 17 October 2023 - 02:49 PM.
#926
Posted 17 October 2023 - 03:03 PM
I don't think so.
It is said neural metabolic rate is similar between various species of mammal, presumably so within bowhead whales too. The brain is 2% of body yet consumes 20% of oxygen, iirc. 10x energy of most other organs. Most of the energy goes towards information transmission function of neurons. If energy production was significantly compromised the neuron would stop functioning. Yet not only have unmodified mice neurons managed to live twice as long when transplanted to rats, and human neurons have lasted over 115 years in humans, but bowhead neurons seem to last for over 200 years. AFAIK bowhead neurons do not seem to have additional protection mechanisms, though maybe they do.
Mitochondrial quality I've heard is maintained via a process of mitoptosis. This is how the neuron, even that of mice, became negligible senescent, able to have high mitochondrial function indefinitely, and some believe could last indefinitely in the right host. I've heard mitoptosis is downregulated with aging. Restoring mitoptosis, I once heard, can even remove negative mutations from the mitochondrial population.
mitoptosis paper
https://www.ncbi.nlm...les/PMC3475017/
One way telomerase therapy might restore youthful function and appearance under the microscope to old cells, even allowing them to produce young tissue, might be via restoring mitoptosis activity.
https://www.ncbi.nlm...les/PMC2912461/
Again reduced clearance and reduced fission proteins in an age related manner. Aging downregulates mitochondrial maintenance mechanisms.
this mitoptosis is what we need... not only in the brain... then trick the other clocks (metilations + telomeres + .....)... for good health we need fresh head and fresh testis....
Edited by HBRU, 17 October 2023 - 03:03 PM.
#927
Posted 17 October 2023 - 03:30 PM
This article is really interesting.
But why is advantageous to kill off older individuals ?!? From egoist gene point of view no sense... just a waste of reproductive potential. We miss something. IMHO the point is we are a MIX of human DNA + mithocondrial DNA + intestine bacterial DNA.... and ALL this DNA want to replicate collaborating. What tears off ? By my opinion is mithocondria. The rest of DNA (the humane one) just is programmed to keep in pace with mitocondrial degenerations.... so changes in both DNA methilations and telometer lengt (and maybe other clocks) are just needed to cope with LESS ATP and much more dirty functioning of mitocondria. This and probably transposons awakening and probably also bad pieces of waste DNA around that spoils the DNA expression. Methilations is an adaptation to other bad factors. Like having a car with brakes broken: you forced to drive slowly... and drive slowly can let you go on.... but problem is not that you go slow, problem is in the broken brakes.
Thanks for reviving some of the old discussions.
It is kind of a chicken and egg argument over whether we are evolved to die off, or if evolution merely uses the already existing aging to tailor the success (or otherwise) of a species.
I am inclined to think that evolution merely makes small adjustments and that we are missing something in the mechanism by which species arise. Hence I can't see humans ever naturally evolving negligible senescence with increasing fertility, say.
So a species could have a different aging mechanism to another - or at least a shortest stick of dynamite, as Bill Andrews phrases it. Pigs and humans both suffer from atherosclerosis, but chimps don't. Different molecular aging mechanisms may underlie this, or it may simply be the way we are put together and the trade offs made at a systems level.
I do not subscribe to any intra-molecular aging mechanism as I once did. I do think such mechanisms could kill us eventually. But I am inclined to think that it is just the natural attrition of cells in the body that causes damage to build up in the surviving cells. The rate of cell loss is naturally different in different species. And it cannot be fully mitigated for by ready made replacement cells and telomerase (although this helps), because the damage is internalised in the surviving cells, and fast cell turnover will just increase the junk that must be dealt with, even as you replace the damaged cells.
#928
Posted 01 November 2023 - 04:00 PM
What is the optimal strategy for slowing telomere loss? It is not telomerase activators
It is with great reluctance that I make this post. Although I remain convinced that telomere shortening with cellular replication imposes a limit on our lifespan, and that an effective telomerase therapy would be an ideal treatment for this, I have come to realise that telomerase activation is not (currently) a valid or cost effective strategy.
There are various molecules purported to activate telomerase, and these have been extensively covered in this thread. I have even tried combining them in various clever ways for maximum synergy. I even took a Lifelength test and found that epitalon was somewhat effective. Nevertheless I have serious concerns about whether telomeres are actually getting longer. I believe that they are not, that we are only reducing the shortening rate (in leukocytes) and that this being the case (it is), there are more effective and cheaper ways of doing this.
First let me cover the point about whether telomeres are actually getting lengthened. This is a oft misunderstood point, and probably the most common question I get from people. If a telomerase activator can increase telomerase to 16% of the level required to maintain telomere length indefinitely, then this should lead to a reduction in the shortening rate by 16%, not to lengthening. Now there is a slight complication to this, as short telomeres are easier to lengthen than long ones (probably because the reduced telomere overhang doesn’t impeded HTERT expression - Shay and Wright showed this can happen on human chromosome 5 [1],[2] - or because a longer overhang somehow impedes the telomerase protein docking with the telomere (my speculation)), so there may be an averaging out effect, and therefore some benefit of telomerase activators offsetting the harm of the shortest telomeres. But we should still see the average telomere length decline. In cell culture this is indeed what is seen (Bill Andrew’s has confirmed this in various talks). And yet people who take TAM818 or cycloastragenol or epitalon report increases in leukocyte telomere length (LTL). How can this be? The answer is rather simple.
Say the white blood cells whose telomeres we are measuring are the progeny of a stem cell with a telomere length of 9000 base pairs (bp). To populate the body with about 4000 WBCs/uL (typical adult level), it will need to split about 24 times. The stem cell itself may only split once, but then each downstream cell splits in turn until there are enough cells.
The telomere length of the 4000 white blood cells in each microlitre of your blood depends on three things: 1) telomere length of the original progenitor, 2) number of divisions, 3) the telomere loss per division. In our example we’ve set the progenitor to have 9kbp, which is pretty long (pristine for a human adult stem cell is about 10k bp). The number of divisions is also fixed - in reality this will change depending on infection status - and this will in real life influence your LTL, which is why it is a good idea to take a telomere test in the summer, or at least the same time each year. But we don’t need to think about this too much in our example. The remaining factor we are left with is the telomere loss per division.
An effective telomerase activator will naturally reduce this. But so will other things. For example Bill Lawrance used epithalamin tablets and over the course of seven years increased his LTL by 27% (6.41kbp to 8.15kbp; I posted about this previously upthread if you want to read more). Using our example stem cell telomere length of 9kbp and 24 divisions, we can work out that his initial 2012 result implied a loss of 107bp/division. Assuming he still has progenitors with 9kbp seven years later, and he has maintained 4000/uL concentration of WBCs in the blood, then the loss per division would have needed to have dropped to 35bp/division to give the new result of 8.15kbp. If we decide his stem cell telomeres will also have gotten shorter in that time, then the loss would be even less than 35bp per division.
And this isn’t an isolated example. Go and have a look at Defytime’s published results on their website (https://defytime.com...omere-analysis/). They’ve had similar results, but over a shorter timespan using TAM818 (admittedly they sometimes measure the change in the shortest 20% telomeres, not the average length, but still..).
So this explains why we can see telomere length increases in downstream cells, with telomerase activators that only slow telomere loss. It also explains why even other lifestyle interventions like meditation, diet or exercise can have mild effects on increasing telomere length with no effect on telomerase expression. It also explains why there is so much variation in the results of telomere tests.
So now we come to the second part of the post. Why telomerase activators aren’t the optimal way to slow telomere loss. The reason is obvious. If all telomerase activators can do is slow loss, then we don’t actually need telomerase activators to do that. We can use other supplements. Let’s look at the evidence.
Nicotinamide increases the number of divisions in human cells by 50% [3]. This is an insane increase! (Bear in mind that Bill Andrews has said TAM818 only gave them about 5% more divisions). But with nicotinamide no telomerase was activated but even so the telomere loss was reduced from 24bp/division to only 9! Now the nicotinamide dose was rather high (5mM), more than we could get in vivo. But we also see similar results from Methylene Blue, using in vivo achievable concentrations (100nM): 20-60% increase in divisions depending on the level of oxygen[4], [5]. Once again, they showed that telomere loss was slowed right down.
The mechanism for slowed telomere loss using either nicotinamide or methylene blue involves increasing the NAD+/NADH ratio, possibly only transiently, in order to improve the functioning of the electron transport chain and control the rise in ROS that normally occurs.
Now before you object that this is all in vitro, we even have evidence that this works in vivo. The NAD+ donor NMN, at a relatively modest dose of 300mg/day increased the LTL of humans by 2-fold [6]! If we feed this into our earlier calculation, this would correspond to reducing the shortening rate by something like 5 times (depending on the actual telomere length values, which aren’t given).
So there we have it. If you want to maintain your telomeres and live longer, the best way to do this is by raising your NAD+ levels. Might it be worth combining doing this with a telomerase activator? Possibly. If telomerase activators are reducing shortening of telomeres by telomerase acting on the telomere itself, then yes, as this is a completely different mechanism to increasing NAD+. But if telomerase activators are reducing shortening by being diverted to the mitochondria, as we know telomerase can do [7], then no.
References
[1] Robin JD, Ludlow AT, Batten K, Magdinier F, Stadler G, Wagner KR, Shay JW, Wright WE. Telomere position effect: regulation of gene expression with progressive telomere shortening over long distances. Genes Dev. 2014 Nov 15;28(22):2464-76. doi: 10.1101/gad.251041.114. PMID: 25403178; PMCID: PMC4233240.
[2] Kim W, Shay JW. Long-range telomere regulation of gene expression: Telomere looping and telomere position effect over long distances (TPE-OLD). Differentiation. 2018 Jan-Feb;99:1-9. doi: 10.1016/j.diff.2017.11.005. Epub 2017 Nov 22. PMID: 29197683; PMCID: PMC5826875.
[3] Kang HT, Lee HI, Hwang ES. Nicotinamide extends replicative lifespan of human cells. Aging Cell. 2006 Oct;5(5):423-36. doi: 10.1111/j.1474-9726.2006.00234.x. Epub 2006 Aug 25. PMID: 16939485.
[4] Atamna H, Nguyen A, Schultz C, Boyle K, Newberry J, Kato H, Ames BN. Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB J. 2008 Mar;22(3):703-12. doi: 10.1096/fj.07-9610com. Epub 2007 Oct 10. PMID: 17928358.
[5] Atamna H, Atamna W, Al-Eyd G, Shanower G, Dhahbi JM. Combined activation of the energy and cellular-defense pathways may explain the potent anti-senescence activity of methylene blue. Redox Biol. 2015 Dec;6:426-435. doi: 10.1016/j.redox.2015.09.004. Epub 2015 Sep 10. PMID: 26386875; PMCID: PMC4588422.
[6] Niu KM, Bao T, Gao L, Ru M, Li Y, Jiang L, Ye C, Wang S, Wu X. The Impacts of Short-Term NMN Supplementation on Serum Metabolism, Fecal Microbiota, and Telomere Length in Pre-Aging Phase. Front Nutr. 2021 Nov 29;8:756243. doi: 10.3389/fnut.2021.756243. PMID: 34912838; PMCID: PMC8667784.
[7] Ale-Agha N, Jakobs P, Goy C, Zurek M, Rosen J, Dyballa-Rukes N, Metzger S, Greulich J, von Ameln F, Eckermann O, Unfried K, Brack F, Grandoch M, Thielmann M, Kamler M, Gedik N, Kleinbongard P, Heinen A, Heusch G, Gödecke A, Altschmied J, Haendeler J. Mitochondrial Telomerase Reverse Transcriptase Protects From Myocardial Ischemia/Reperfusion Injury by Improving Complex I Composition and Function. Circulation. 2021 Dec 7;144(23):1876-1890. doi: 10.1161/CIRCULATIONAHA.120.051923. Epub 2021 Oct 21. PMID: 34672678.
So Turbuckle fission part of protocol can also be usueful to make cells replicate without loosing to much telomere lenght..?!? maybe just adding something cheap and simple as Gotu Kola can add the benefit of division without TELOMERE ATTRITION (or with little). I also personally add to this "fission" part Fisetin, Resveratrol and 2 mg of Copper. Copper + Resveratrol to clean out bad DNA around, Fisetin as senolythic. Cell division boost should help the immunitary system to get rid of bad DNA, bad cells. Its a lot oxidizing. But I do this once a week max. The rest of the week I stay into fission (eat chocolate, take glycerol mono stearate, take antioxidants and such) + take pravastatin and small quantity of telmisartan.... COPPER important as it makes cells differentiate. So replication of both cells and mithocondria and differentiation occur during "fission" (also importanto to eat well in this time). Stem cells indifferentiation increase during "fusion"... and here can be done some fasting.
Role of Copper on Mitochondrial Function and Metabolism - PMC (nih.gov)
Edited by HBRU, 01 November 2023 - 04:36 PM.
#929
Posted 01 November 2023 - 04:48 PM
to help differentiation and to help eliminating bad mitocondria -perhaps- also vitamin A useful once a week... but this have not done yet. Copper + Vitamin A + Reveratrol + Fisetin ... with lots of nicotinammide :D---- maybe too much
Vitamin A supplementation increased mitochondrial superoxide anion radical () production (Table 3) and induced lipid peroxidation, protein carbonylation and nitration, and oxidation of protein thiol groups in mitochondrial membranes isolated from rat cerebral cortex, cerebellum, substantia nigra, striatum, frontal cortex, and hypothalamus [67–69, 76, 78].
Vitamin A and Retinoids as Mitochondrial Toxicants (hindawi.com)
Edited by HBRU, 01 November 2023 - 04:50 PM.
#930
Posted 02 November 2023 - 08:59 AM
I read that iron is antagonistic to copper, and when iron gets high, copper can go low; and copper deficiency is known to result in gray or white hair. Copper important for cell differentiation. So IP6 and EDTA to load off iron, and maybe some Copper supplement -some time- seems a good idea.
Iron overload can induce mild copper deficiency - PubMed (nih.gov)
Also tagged with one or more of these keywords: telomeres, nad, nampt, ampk, resveratrol, allicin, methylene blue, nmn, sirtuins, statin
2 user(s) are reading this topic
0 members, 2 guests, 0 anonymous users


















































